Description
The instruction for medical use
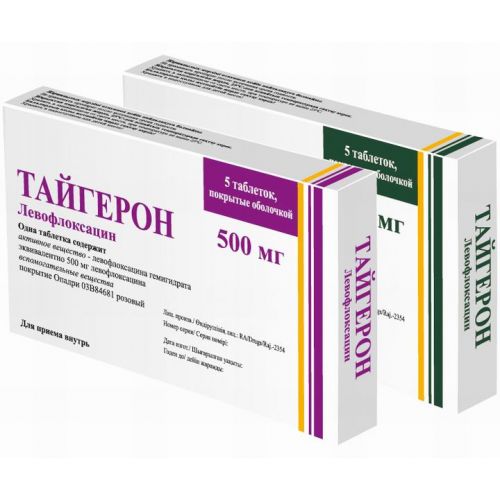
of TAYGERON® medicine
the Trade name
of Taygeron®
the International unlicensed
name Levofloxacin Dosage Form
of the Tablet, film coated, 500 mg and 750 mg
Structure
One tablet contains
active agent – levofloksatsina gemigidrat is equivalent a levofloksatsina of 500 mg or 750 mg,
excipients: cellulose microcrystalline (avitset PH 102) K-30 povidone, krospovidon, magnesium stearate, silicon dioxide colloidal anhydrous
structure of a cover: Opadry 03B84681 pink [gipromeloz (E 464), polyethyleneglycol/macrogoal, gland (III) oxide red (E 172), gland (III) oxide yellow (E 172), titan dioxide (E 171)].
The description
of the Tablet of a capsulovidny form, film coated pink color, with stamping 500 (for a dosage of 500 mg) or 750 (for a dosage of 750 mg) on one party.
Pharmacotherapeutic group
Antimicrobial drugs – derivatives of a hinolon. Ftorkhinolona. Levofloxacin. The code of automatic telephone exchange J01MA12
the Pharmacological
Pharmacokinetics Later properties of intake levofloxacin is quickly and completely soaked up from the digestive tract (DT), the absolute bioavailability of tablets of 500 mg and 750 mg of a levofloksatsin is 99%. Cmax is reached in 1–2 hours after reception.
At a concomitant use with food time of achievement of Cmax slightly increases (by 1 h) and slightly Cmax (by 14%) therefore it is possible to appoint levofloxacin regardless of meal decreases.
The pharmacokinetics of a levofloksatsin in/in and oral introduction has later no essential distinction, has linear character and is predictable at single and repeated introduction inside and in / century.
The plasma profile of concentration of a levofloksatsin in/in introductions is similar later to that after intake in an equivalent dose. Later in/in single introductions in a dose of 500 mg (infusion within 60 min.) Cmax makes 6.2±1.0 mkg/ml, at a dose of 750 mg (infusion within 90 min.) — 11.5±4.0 mkg/ml. Constant concentration in plasma is reached in 48 hours at reception of 500-750 mg of 1 times a day. At repeated introduction to healthy volunteers of Cmax value made: at oral introduction of 500 mg/days — 5.7±1.4 mkg/ml, 750 mg/days — 8.6±1.9 mkg/ml
the Average volume of distribution is 74–112 l after single and repeated introductions of doses of 500 mg and 750 mg. It is widely distributed in body tissues, well gets into tissue of lungs (concentration in lungs is 2-5 times higher than concentration in plasma). In vitro in the range of the concentration corresponding to clinical values (1–10 mkg/ml), linking with proteins of plasma (mainly with albumine) makes 24–38% and does not depend on concentration of a levofloksatsin. Stereokhimicheski is stable in plasma and in urine, does not turn in the enantiomer, D-ofloxacin.
In an organism it is practically not metabolized. It is removed mainly in not changed view with urine (about 87% of a dose during 48 h), insignificant quantities — with excrements (less than 4% for 72 h). Less than 5% are defined in urine in the form of the metabolites (desmetit, nitrogen oxide) having insignificant specific pharmacological activity. T1/2 makes 6–8 hours after single or repeated introductions inside or in / century.
The general clearance is 144–226 ml/min., renal clearance — 96–142 ml/min., excretion is carried out by glomerular filtration and canalicular secretion. Simultaneous use of Cimetidinum or a probenetsid leads to decrease in renal clearance respectively by 24 and 35% that demonstrates secretion of a levofloksatsin by proximal departments of tubules. Crystals of a levofloksatsin in fresh-gathered urine are not found.
Patients with a renal failure
the Renal failure influences pharmacokinetics of a levofloksatsin. At decrease in renal function removal by kidneys and clearance decrease, elimination half-life increases, as shown in the table below.
clearance of creatinine (ml/min.)
& lt,
20 20-40 50-80
clearance of a levofloksatsin (ml/min.)
13 26 57
elimination half-life (hour)
35 27 9
Patients of advanced age
of Essential differences in kinetics of a levofloksatsin between patients there is no young and advanced age, except for the distinctions connected with clearance of creatinine.
A pharmacodynamics
Levofloxacin (fluorine-2, metil-10-(1-piperazinit)-oxo-7H-piridol [1,2,3,-]-1.6-carboxyl acid) – synthetic antibacterial agent of group of ftorkhinolon. Has a wide range of antibacterial action. The fast bactericidal effect is provided with oppression levofloksatsiny bacterial DNK-girazy enzyme which belongs to type topoisomerases II. The volume structure of DNA of bacteria is as a result broken and their division is blocked. The range of activity of a levofloksatsin includes gram-positive and gram-negative bacteria, including nonfermentative bacteriums which often cause a nozokomialny infection and also atypical microorganisms, such as S. of pneumoniae, S. of trachomatis, M. pneumoniae, L. pneumophila, Ureaplasma. Besides, pylori such activators as mycobacteria, N. are sensitive to a levofloksatsin and anaerobe bacterias.
Are sensitive to drug: gram-positive aerobes – Enterococcus faecalis, Staphylococcus aureus metitsillinchuvstvitelny, Staphylococcus haemolyticus metitsillinchuvstvitelny, Staphylococcus saprophyticus, Streptococci C, G, Streptococcus agalactiae, Streptococcus pneumoniae, including resistant to penicillin, Streptococcus pyogenes, gram-negative aerobes – Acinetobacter baumannii, Citrobacter freundii, Eikenella corrodens, Enterobacter agglomerans, Enterobacter cloacae, Escherichia coli, Haemophilus influenzae, including resistant to ampicillin, Haemophilus parainfluenzae, Klebsiella oxytoca, Klebsiella pneumoniae, Moraxella catarrhalis including producing α-lactamelements, Morganella morganii, Pasteurella multocida, Proteus mirabilis, Proteus vulgaris, Providencia rettgeri, Providencia stuartii, Pseudomonas aeruginosa, Serratia marcescens, anaerobe bacterias – Bacteroides fragilis, Clostridium perfringens, Peptostreptococcus, others – Chlamydia pneumoniae, Chlamydia psittaci, Legionella pneumophila, Mycoplasma pneumoniae.
Are non-constantly sensitive to drug: gram-positive aerobes – Staphylococcus haemolyticus metitsillinrezistentny, gram-negative aerobes – Burkholderia cepacia, anaerobe bacterias – Bacteroides ovatus, Bacteroides thetaiotamicron, Bacteroides vulgaris, Clostridium difficile.
To rezistentna drug: gram-positive aerobes – Staphylococcus aureus, metitsillinrezistentny.
As well as other ftorkhinolona, levofloxacin it is not active concerning spirochetes.
Indications
– acute sinusitis, exacerbation of chronic bronchitis, extra hospital pneumonia
– the complicated and uncomplicated infections of an urinary path (including pyelonephritis)
– chronic bacterial prostatitis
– infections of skin and soft tissues
– septicaemia/bacteremia
– an intraabdominal infection
accept the Route of administration and doses of the Tablet Taygeron® 1–2 times a day. The dose depends on type and weight of an infection. Duration of treatment depends on the course of the disease and is no more than 14 days. It is recommended to continue treatment for 48–72 hours after normalization of body temperature or the destruction of activators confirmed with microbiological tests.
The tablets Taygeron® should be swallowed, without chewing, washing down with enough liquid. For convenience of dosing a tablet it is possible to divide with the help risks for division. A pill can be taken both together with food, and in other time.
The recommended doses for adult patients with normal function of kidneys (clearance of creatinine & gt, 50 ml/min.)
Indications
the Daily dose
the Number of inclusion
in days.
Prodolzhitel
a treatment nost
Acute sinusitis
of 500 mg
of 1 times
of 10-14 days
Exacerbation of chronic bronchitis
of 250-500 mg
of 1 times
of 7-10 days
Extra hospital
pneumonia
of 500 mg
1-2 times
of 7-14 days
Uncomplicated infections of an urinary path
of 250 mg
of 1 times
3 days
Chronic bacterial prostatitis
of 500 mg
of 1 times
of 28 days
the Complicated infections of an urinary path, including pyelonephritis
of 250 mg
of 1 times
of 7-10 days
of the Infection of skin and soft tissues
of 250-500 mg
1–2 times
of 7-14 days
Septicaemia / bacteremia
of 500 mg
1–2 times
of 10-14 days
the Intraabdominal infection *
500 mg
of 1 times
of 7-14 days
* In a combination with the antibiotics operating on anaerobic activators.
Dosing for patients with impaired renal function
(clearance of creatinine & lt, 50 ml/min.)
Clearance of creatinine
the dosing Mode (depending on weight of an infection)
50–20 ml/min.
the first dose of 250 mg
following:
125 mg / 24 hour
the first dose: 500 mg
following:
250 mg / 24 hour
the first dose: 500 mg
following:
250 mg / 12 hour
of 19-10 ml/min.
the first dose: 250 mg
following:
125 mg / 48 hours
the first dose: 500 mg
following:
125 mg / 24 hour
the first dose: 500 mg
following:
125 mg / 12 hour
& lt, 10 ml/min., (and also at a hemodialysis and HAPD1)
the first dose: 250 mg
following:
125 mg / 48 hours
the first dose: 500 mg
following:
125 mg / 24 hour
the first dose: 500 mg
following:
125 mg / 24
1 After a hemodialysis or the chronic out-patient peritoneal dialysis (COPPD) additional doses are not necessary hour.
Side effects
Often
– lack of appetite, nausea, vomiting, an abdominal pain, a meteorism, digestive disturbances, diarrhea, a constipation
– increase in activity of liver enzymes (ALT, nuclear heating plant) and bilirubin level in blood serum
Sometimes
– skin rash, an itching, erubescence
– a headache, dizziness, drowsiness, insomnia, an asthenia
– increase in level of creatinine in blood serum
– an eosinophilia, a leukopenia
Seldom
– urticaria, a spasm of bronchial tubes, short wind
– a melena
– paresthesias in brushes, a tremor, feeling of concern, a condition of fear, a spasm, confusion of consciousness
– tachycardia, a lowering of arterial pressure
– damage of sinews, including a tendinitis, joint or muscles pain
– a neutropenia, the thrombocytopenia resulting in the increased tendency to hemorrhages or bleedings
Is very rare
– a Quincke’s edema, an acute anaphylaxis, anaphylactoid reactions
– hypotonia, shock, collapse
– lengthening of a QT interval
– a renal failure, an acute renal failure, interstitial nephrite
– pseudomembranous colitis, a coloenteritis
– a photosensitization
– a hypoglycemia
– disorder of vision and hearing, flavoring sensitivity and sense of smell, a hypesthesia
– psychotic reactions, in the form of hallucinations and a depression, including suicide thoughts
– a rupture of sinews for example, a rupture of an Achilles tendon (it can be shown for 48 hours from an initiation of treatment and affect both extremities)
– hepatitis
– an agranulocytosis
– fever, an allergic pneumonitis, a vasculitis
– the muscle weakness which is of particular importance for patients with a heavy myasthenia
In isolated cases
– porphyria attacks
– Stephens’s syndrome – Johnson, the toxic epidermal necrolysis (Lyell’s disease) and an exudative mnogoformny erythema
– a rhabdomyolysis
– extrapyramidal symptoms and other disturbances of muscular coordination
– hemolytic anemia, a pancytopenia
– candidiasis, development of resistance of microorganisms
of the Contraindication
– hypersensitivity to a levofloksatsin or other hinolona – epilepsy and other damages of the central nervous system with the lowered convulsive threshold
– the damages of sinews connected with use of hinolon in the anamnesis
– pregnancy and the period of a lactation
– children’s and teenage age up to 18 years
Medicinal interactions
Absorption of a levofloksatsin significantly decreases at simultaneous use with the antacids containing magnesium and aluminum and also with preparations of iron, the sukralfat. The recommended span between reception of Taygerona® and the specified drugs has to be not less than 2 hours.
The pharmacokinetics of a levofloksatsin did not change in clinically significant degree when TaygeronÒ was applied together with the following medicines: calcium carbonate, digoxin, glibenclamide, ranitidine.
Impact of other medicines on TaygeronÒ Theophylline, fenbufen and other similar non-steroidal anti-inflammatory drugs (NPVS)
– simultaneous use of hinolon with the theophylline, NPVS and other drugs reducing a convulsive threshold can lead to the significant decrease in a cerebral convulsive threshold, despite the lack of pharmacokinetic interactions of TaygeronaÒ with theophylline. In the presence of a fenbufen the concentration of TaygeronaÒ increases almost by 13%.
Probenetsid and Cimetidinum
– probenetsid and Cimetidinum make considerable impact on removal of TaygeronaÒ. Cimetidinum and probenitsid reduce renal clearance of a levofloksatsin (by 24% and 34% respectively) as both drugs can block canalicular secretion of a levofloksatsin in kidneys. At the simultaneous use of medicines of type of a probenitsid and Cimetidinum influencing canalicular secretion of kidneys, treatment of TaygeronomÒ should be carried out with care, especially at disturbance of renal function.
Impact of TaygeronÒ on other medicines
Cyclosporine
– elimination half-life of cyclosporine increases by 33% at its simultaneous use with TaygeronomÒ.
Antagonists of vitamin K
– treatment of TaygeronomÒ in a combination with the antagonist of vitamin K (for example, with warfarin) can lead to increase in indicators of coagulative tests (the prothrombin time (PT) taking into account the international normalized relation (INR)) and/or to strengthening of bleeding which can take the serious form. Therefore, monitoring of coagulative tests is necessary for the patients receiving antagonists of vitamin K
Glucocorticoids increase risk of a rupture of sinews.
The medicines extending QT interval
– the medicines capable to prolong QT interval (for example, antiarrhythmic means of the classes IA and III, tricyclic antidepressants, macroleads) should be applied with care at simultaneous use with TaygeronomÒ (see. Special instructions).
Use of a levofloksatsin along with alcohol is not recommended.
Special instructions
In the most hard cases of pneumococcal pneumonia of TaygeronÒ it can be not the most optimum therapy.
The tendinitis is in rare instances possible, is more often than the Achilles tendon that can lead to its gap. Risk of developing a tendinitis and a rupture of a sinew is increased at advanced age and at the patients using corticosteroid drugs. Strict observation of such patients when assigning of TaygeronaÒ with it is necessary. All patients at emergence of symptoms of a tendinitis need to see the attending physician. At suspicion of a tendinitis treatment of TaygeronomÒ it is necessary to stop and begin immediately the corresponding treatment of the affected sinew (for example, an immobilization).
Patients with predisposition to spasms
of TaygeronÒ it is contraindicated to patients with epilepsy in the anamnesis. As well as in cases with other hinolona, it is necessary to observe the maximum care in case of the patients inclined to epileptic seizures, for example, of patients with already available damages of the central nervous system, at simultaneous treatment fenbufeny and similar, the non-steroidal anti-inflammatory drugs or drugs lowering a cerebral convulsive threshold such as theophylline (see. Medicinal interactions). In case of convulsive spasms the treatment of TaygeronomÒ needs to be stopped.
Patients with deficit glyukozo-6-fosfatdegidrogenazy
Patients with the latent or demonstrating insufficient activity glyukozo-6-fosfatdegidrogenazy can be prone to hemolytic reactions at treatment by antibacterial hinolona therefore it is necessary to be careful at use of TaygeronÒ.
Combined use of any antibacterial agents can lead to the disturbances connected with their influence on normal microflora. Consecutive infection which will demand additional treatment can develop.
Patients with a renal failure
Due to the removal of a levofloksatsin mainly with kidneys, it is necessary to carry out dose adjustment of TaygeronaÒ for patients with a renal failure (see the section Route of Administration and Doses).
Hypersensitivity reactions
of TaygeronÒ the Quincke’s disease can cause serious, potentially lethal hypersensitivity reactions, for example, up to an acute anaphylaxis, sometimes after the first dose (see. Side effects). Patients should stop immediately treatment and to see the attending physician or the emergency doctor which will take the appropriate emergency measures.
The hypoglycemia
At use of TaygeronaÒ, as well as in case of all hinolon, can arise a hypoglycemia, usually, at patients with the diabetes which was at the same time on treatment by oral hypoglycemic means, for example, glibenclamide or insulin. Such patients are recommended to carry out strict monitoring of level of glucose to blood (see. Side effects).
Prevention of a photosensitization
In spite of the fact that at treatment of TaygeronomÒ the photosensitization meets seldom, the patient is recommended not to subject itself without need to impact of strong sunlight or artificial ultraviolet radiation, for example, sunlight lamps, a sunbed, in order to avoid a photosensitization.
The patients who are on treatment by antagonists of vitamin K
Due to the possible increase in indicators of coagulative tests (prothrombin time / MNO) and/or strengthening of bleedings at the patients who are on treatment of TaygeronomÒ in a combination with the antagonist of vitamin K (for example, warfarin), it is necessary to control fibrillation indicators when assigning simultaneous use of these drugs (see. Medicinal interactions).
Psychotic reactions
At the patients applying hinolona, including TaygeronÒ were registered psychotic reactions. Seldom or never they progressed before emergence of thoughts of suicide and behavior with drawing to themselves mutilations, sometimes even after the unique dose of TaygeronaÒ (see. Side effects). In case of development in the patient of psychotic reactions of TaygeronÒ it is necessary to cancel and start the appropriate measures. It is recommended to be careful if TaygeronÒ has to appoint the patient with psychoses or with a mental disease in the anamnesis.
Lengthening of an interval of QT
It is necessary to be careful when using ftorkhinolon, including TaygeronÒ, in case of patients with the known risk factors of lengthening of an interval of QT, such as, for example:
– a congenital syndrome of the extended QT
– simultaneous use of the drugs known for the ability to prolong QT interval (for example, antiarrhythmic means of the classes IA and III, tricyclic antidepressants, macroleads), (see. Medicinal interactions)
– not adjusted disturbance of electrolytic balance (for example, a hypopotassemia, a hypomagnesiemia)
– advanced age
– a heart trouble (for example, heart failure, a myocardial infarction, bradycardia), (see. Side effects, Overdose).
Peripheral neuropathy.
It is necessary to cancel TaygeronÒ if the patient has neuropathy symptoms, in order to avoid development of an irreversible state.
Opiates.
Definition of opiates in urine at sick, treated TaygeronomÒ, can yield in a false manner positive take. Can be required to confirm positive take on opiates with other, more specific method.
Disturbances of a liver and biliary tract.
It is necessary to advise patients to stop treatment and to see the doctor if they have signs and symptoms of a disease of a liver, such as anorexia, jaundice, dark coloring of urine, itching, morbidity of a stomach.
Pregnancy and period of a lactation.
Pregnant women and the feeding women cannot take the pill Taygerona due to the lack of these clinical trials and possible risk of defeat of a ftorkhinolonama of the cartilaginous tissue of the growing organism bearing loading.
Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
Some side effects, for example, dizziness, drowsiness, disorders of vision, can reduce ability of the patient to concentration of attention and speed of its reactions that can constitute danger in situations where such abilities are especially important (for example, when driving and working mechanisms).
Overdose
Symptoms: nausea, dizziness, confusion of consciousness, loss of consciousness, attacks of spasms, erosive injuries of a mucous membrane of digestive tract, lengthening of an interval of QT.
Treatment: it is necessary to make careful observation of the patient, including the ECG. In cases of obvious overdose the gastric lavage is appointed. Symptomatic treatment. Mucous a stomach apply antiacid means to protection. The hemodialysis, including peritoneal dialysis, are not effective for removal of a levofloksatsin from an organism. Specific antidote is absent.
Forms of release and packing
On 5 or 10 tablets in blister strip packaging from a film of polyvinylchloride and aluminum foil.
On 1 blister strip packaging together with the instruction for medical use in the state and Russian languages place in a cardboard pack.
To Store storage conditions in the place protected from light at a temperature not above 25 °C.
To store out of children’s reach.
3 years
not to use a period of storage after the termination of the expiration date specified on packing!
Prescription status
According to the prescription
the Producer Kusum Heltker Pvt.
Ltd., JV 289 (A), Riiko Indl. Chopanki’s Area, Bkhivadi (Raj.), India
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods):
Dr_-Pharm, (Kazakhstan) LLP, Republic of Kazakhstan,
Almaty, ave, Dostyk, 117/6, BC Khan Tengri,
ph. / fax: 295-26-50, 295-26-55
To develop
Additional information
| Ingredient |
|---|















Reviews
There are no reviews yet.