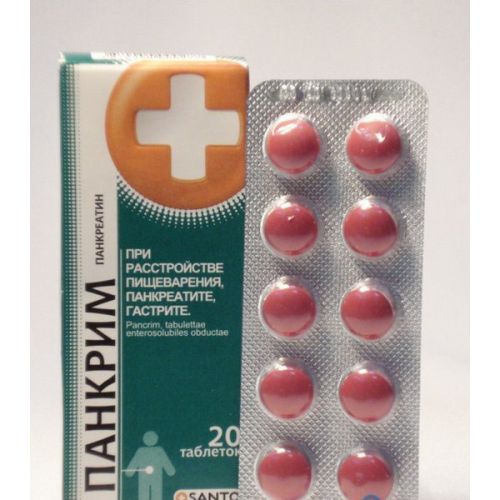
Description
The instruction
for medical use
of PANKRIM® medicine
the Trade name
of Pankrim®
the International unlicensed name
Is not present
the Dosage form
of the Tablet, covered with a kishechnorastvorimy cover
Structure
One tablet contains
active agent – Pancreatinum 250.000
excipients of lactose monohydrate, polyvinylpirrolidone, polysorbate 80, talc, calcium stearate,
structure of a cover: an atsetilftaliltsellyuloza, the titan dioxide (E 171), liquid paraffin, triacetin, Ponso dye 4R (E 124)
the Description
of the Tablet, coated, red color, with a biconvex surface, with a specific smell, On cross section are visible two layers.
Pharmacotherapeutic group
Drugs, digestant (including fermental drugs).
The code of automatic telephone exchange A09AA02
the Pharmacological
Pharmacokinetics Enzymes properties – the natural components promoting digestion of food. Tablets, film coated, protect active enzymes from influence of hydrochloric acid of a stomach. A small part of enzymes is allocated with excrements.
The maximum enzymatic activity of drug is noted in 30-45 min. after oral administration.
The pharmacodynamics
of Pankrim® fills insufficiency of vneshnesekretorny function of a pancreas. The pancreatic enzymes which are a part promote the proteolysis to amino acids, fats – to glycerin and fatty acids, starch – to dextrins and monosaccharides.
Панкрим® improves a functional condition of digestive tract, normalizes digestion processes. Trypsin inhibits stimulated secretion of a pancreas. Pancreatic enzymes are released from a dosage form in the alkaline environment of a small intestine since are protected from effect of gastric juice by a cover. Панкрим® can reduce pain, having analgeziruyushchy effect in chronic pancreatitis and has bile-expelling effect that is caused by protease influence.
Indications
For replacement therapy at
– insufficiency of vneshnesekretorny function of a pancreas
– chronic pancreatitis in a remission stage
– states, after a resection of a stomach, a pancreas, intestines,
followed by disturbances of digestion of food, a meteorism, diarrhea
(as a part of combination therapy)
– disturbance of digestion at patients with normal function of a gastro
intestinal path in case of an error in food and also at disturbances
of chewing function, the forced long immobilization,
inactive lifestyle
– intestines diseases, the advances
of intestinal contents which are followed by disturbance
– diseases of a liver, gall bladder,
the zhelchevydeleniye which are followed by disturbance
– preparation to radiological and to ultrasound examination
of abdominal organs
to Accept the Route of administration and doses, without chewing, with enough liquid.
It is impossible to wash down with alkaline mineral water.
The dose of drug is defined individually depending on extent of disturbance of digestion at meal time or after a meal.
Adults and teenagers are more senior than 14 years – 250-500 mg (1-2 tablets), if necessary the dose can be increased.
The maximum daily dose – 4 g.
For children of 6-14 years the single dose makes 250 mg.
Duration of a course of treatment is determined by the attending physician.
Side effects
Seldom
– diarrhea, discomfort in epigastric area
– allergic reactions (skin rash, an itching)
Are possible
– development a hyper uricosuria, increase in level of uric acid in
blood plasma (at prolonged use in high doses)
– development of strictures (fibrous colonopathy) in ileocecal department and in
the ascending part of a colon in a mucoviscidosis in case of exceeding
a necessary dose of Pankrima®
Is very rare
– nausea, vomiting, a constipation
– irritation of a mucous membrane of an oral cavity at children, perianal
irritation (at use of Pankrima® in high doses)
Contraindications
– hypersensitivity to drug components
– exacerbation of chronic diseases of digestive tract,
chronic pancreatitis
– acute pancreatitis
– diabetes
– hereditary intolerance of a galactose, a lactose intolerance,
a sprue
– children’s age up to 6 years
Medicinal interactions
At simultaneous use of Pankrima® with iron preparations and folic acid the decrease in absorption of iron and folic acid is possible. Simultaneous use of Pankrima® and antiacid means containing calcium a carbonate and/or magnesium hydroxide can lead to decrease in efficiency of Pancreatinum.
There can be a decrease in efficiency of drug at its simultaneous use to blockers of H2-histamine receptors, inhibitors of the protonew pump.
At reception of Pankrima® the reduction of efficiency of hypoglycemic drugs is possible.
Special instructions
At emergence of side effects: administration of drug it is necessary to stop and consult with your attending physician.
It is not necessary to appoint in high doses to children up to 15 years the suffering mucoviscidosis owing to increase in risk of development of strictures (fibrous colonopathy).
Pregnancy and the period of a lactation
Safety of use of Pankrima® at pregnancy and in the period of a lactation is not established. Use is possible in cases when the expected advantage for pregnant women and the nursing women exceeds potential risk for a fruit or the child.
The feature of influence of medicine on ability to run the vehicle or potentially dangerous
Pankrim® mechanisms does not affect ability to run the vehicle or potentially dangerous mechanisms.
Overdose
Symptoms: hyper uricosuria, hyperuricemia. Children have a constipation (at use of Pankrima® in high doses).
Treatment: medicine cancellation, symptomatic therapy.
A form of release and packing
On 10 tablets in blister strip packaging from a film of polyvinylchloride and aluminum foil.
On the 2nd blister strip packagings together with the instruction for medical use in the state and Russian languages place in a pack from cardboard together with the instruction for medical use in the state and Russian languages.
To Store storage conditions in dry, protected from light, the place, at a temperature not above 25 °C.
To store out of children’s reach!
2 years
not to apply a period of storage after the expiry date specified on packing.
Prescription status
Without prescription
JSC Khimpharm Producer,
REPUBLIC OF KAZAKHSTAN, Shymkent, Rashidov St.,/N, ph.: 561342
The address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods)
of JSC Khimpharm,
Shymkent, REPUBLIC OF KAZAKHSTAN, Rashidov St.,/N, ph.: 560882
Phone number 7252 (561342)
Fax number 7252 (561342)
E-mail address of standart@santo.kz
Additional information
| Ingredient |
|---|



















Reviews
There are no reviews yet.