Description
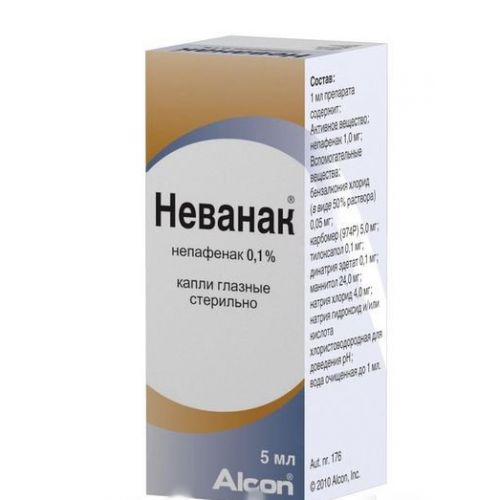
Trade name
of Nevanac ®™
the International unlicensed
name Nepafenacum Lekarstvennaya
the Drop form eye, 5 ml
Structure
of 1 ml of drug contains
active agent: Nepafenacuma of 1.0 mg
excipients: a benzalkoniya chloride, carbomer (974 P), tiloksapol, dinatrium edetat, Mannitolum, sodium chloride, acid chlorohydrogen and/or sodium hydroxide (for correction rn), the water purified.
The description
Uniform suspension from light yellow till light orange color.
Pharmacotherapeutic group
Drugs for treatment of diseases of eyes. Non-steroidal anti-inflammatory drugs. Nepafenacum.
The ATX S01BC10 code
the Pharmacological
Pharmacokinetics Later properties of instillation in eyes average concentration in
Cmax plasma make 0.310 ± 0.104 ng/ml of a Nepafenacum and 0.422 ± 0.121 ng/ml of an amfenak.
Nepafenacum is exposed to rather fast bioactivation with formation of an amfenak under the influence of intraocular hydrolases. Then amfenak is exposed to extensive metabolism with formation of more polar metabolites by means of hydroxylation of an aromatic ring that leads to formation of glyukuronidny conjugates. The radio stratographic analysis which is carried out before hydrolysis with participation of β-glucuronidase shows that all metabolites are in a form of glyukuronidny conjugates, except for an amfenak.
The main metabolites in plasma are amfenak which concentration at Cmax makes about 13% and 5 hydroxies Nepafenacum which concentration at Cmax makes about 9%.
A pharmacodynamics
Nepafenacum is a predecessor of the nonsteroid medicine having anti-inflammatory and soothing properties. After instillation in eyes, Nepafenacum gets into a cornea and non-steroidal anti-inflammatory drug will be transformed by hydrolysis in eye tissues in amfenak. Amfenak inhibits effect of prostaglandin-H-synthase (cyclooxygenase), the enzyme necessary for production of prostaglandin.
Indications
– prevention and treatment of postoperative pain and the inflammation connected with surgery on removal of a cataract
– reduction of risk of the postoperative macular hypostasis connected with cataract surgery at diabetic patients
the Route of administration and doses
For instillation in eyes.
To stir up a bottle before drug use.
For prevention and treatment of postoperative pain and inflammation the dose sostavyat 1 drop of drug in a conjunctival sac of the affected eye (eyes) 3 times a day, beginning in 1 day prior to cataract surgery, and up to 21 days of the postoperative period. In 30-120 minutes prior to operation it is necessary to dig an additional drop.
For reduction of risk of the postoperative macular hypostasis connected with cataract surgery at diabetic patients, the dose sostavyat 1 drop of drug in a conjunctival sac of the affected eye (eyes) 3 times a day, beginning in 1 day prior to surgery of a cataract and up to 60 days of the postoperative period. In 30-120 minutes prior to operation it is necessary to dig an additional drop.
Considering that after topical ophthalmologic administration the system absorption minimum, is absent need of correction of a dose at patients with diseases or abnormal liver functions and kidneys.
At use more than one local ophthalmologic medicine, an interval between use of drugs has to make not less than 5 minutes.
It is not necessary to touch with a bottle dropper tip eyes or any other surface to avoid pollution of a tip of a dropper and contents of a bottle.
After use it is recommended to close densely a bottle a cover.
Side effects
Local
Infrequently:
– a keratitis
– an iritis
– a choroidal exudate
– deposits in a cornea
– eye pain
– a photophobia
– discomfort in eyes, feeling of a foreign body in an eye
– ambiguity of sight
– xerophthalmus
– discharges from eyes
– allergic conjunctivitis, an itching of eyes
– formation of crusts on an edge of the lid
– increase in dacryagogue
– conjunctiva hyperaemia
System
Infrequently:
– the headache
– nausea
– hypersensitivity
-extensibility of skin (dermatoliz Alibera)
the Frequency and gravity of the following side reactions cannot be determined by the reason of a nedostochnost of data.
Local: delay of recovery of a cornea, defect of a corneal epithelium, turbidity of a cornea, scarring of a cornea, decrease in visual acuity, irritation of eyes, eye hypostasis.
System: dizziness, increase in pressure of blood.
Side reactions from eyes can be found in diabetic patients with higher level of frequency in comparison with other patients.
Contraindications
– hypersensitivity to any of drug components, or to other non-steroidal anti-inflammatory drugs (NSPVP)
– the patients with asthma attacks, a small tortoiseshell or acute rhinitis accepting acetylsalicylic acid for stopping of attacks of disease or others
Medicinal interactions
Interaction with other medicines is improbable NSPVP.
There are very limited data on sharing of analogs of prostaglandin and the drug Nevanac ®™ of a drop eye. Considering mechanisms of their action, sharing of the specified medicines is not recommended.
Special instructions
during the Nevanac ®™ drug treatment of a drop eye it is recommended to avoid impact of sunlight.
In case of purpose of a Nepafenacum the diabetic patients after cataract surgery for the purpose of prevention of macular hypostasis at a nalichiniya of an accessory factor of risk need careful assessment of a ratio advantage/risk and more attentive observation of the patient.
Local ophthalmologic use of NSPVP can lead ment of a keratitis. Long use of NSPVP can cause a rupture of cells of an epithelium, thinning of a cornea, a cornea erosion, ulceration on a cornea in some predisposed patients or perforation of a cornea that can create risk of loss of sight. Patients with signs of a rupture of cells of an epithelium of a cornea have to stop immediately the Nevanac ®™ drug treatment of a drop eye and be under control of a condition of a cornea.
Prolonged topical ophthalmologic use of NSPVP can increase risk of emergence and severity of side reactions from a cornea and to create threat of loss of sight.
NSPVP of external use can slow down or delay recovery process. Simultaneous use of NSPVP of external use and steroids of external use can increase risk of emergence of problems of recovery.
Local ophthalmologic have to be applied by NSPVP with care at treatment of patients with complications after surgeries in the eyes, cornea denervation, defects of an epithelium of a cornea, diabetes, superficial diseases of eyes (for example, with a xerophthalmus syndrome), a pseudorheumatism or the repeated eye surgeries performed during a short period.
There is a risk of developing of intensive bleeding in eye tissues (including hyphemas) at use of NSPVP in the form of eye drops in combination with surgery in the eyes. Неванак™ drops eye it has to be applied with care at the patients having a predrapolozhennost to bleeding in the anamnesis or taking other medicines prolonging a blood clotting time.
Chloride the benzalkoniya which is usually used as preservative in drugs for treatment of eyes can cause a dot keratitis and/or a toxic ulcer keratitis. As Nevanac ®™ of a drop eye contains chloride a benzalkoniya, careful medical observation of a condition of patients at repeated or long use of drug is required.
Use of local anti-inflammatory drugs can mask symptoms of an acute infection of an eye. NSPVP have no antimicrobic properties. In case of development of an eye infection use of NSPVP with antibacterial agents has to be carried out with observance of precautionary measures.
At drop drug Nevanac ®™ use eye there is a possibility of development of cross sensitivity to acetylsalicylic acid, derivatives of fenilatsetilovy acid and also other NSPVP.
The pediatrics
Safety and efficiency of use of the drug Nevanac ®™ at children is not established.
Pregnancy
of Adequate data on drop drug Nevanac ®™ use eye at pregnant women it is not received. As system influence at the Nevanac ®™ drug treatment of a drop eye is insignificant, the risk on a course of pregnancy can be considered low. However, as the inhibition of synthesis of prostaglandin can negatively influence pregnancy course, and/or development of an embryo/fruit, and/or childbirth, and/or development of the child in the post-natal period, Nevanac ®™ of a drop eye it is not recommended to use during pregnancy and women with the genital potential which is not using contraception.
A lactation
Considering that system influence of the drug Nevanac ®™ of a drop eye on an organism of the feeding woman slightly, this drug can be used during feeding by a breast.
Eye chloride a benzalkoniya which can cause irritation of eyes contains contact lenses of Nevanac ®™ of a drop and, as we know, decolours soft contact lenses. Besides, carrying contact lenses is not recommended during the postoperative period after surgery concerning a cataract. Therefore it is recommended to refrain from carrying soft contact lenses during the Nevanac ®™ drug treatment of a drop eye.
Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
As well as with other eye drops, after use of drug the temporary ambiguity of sight or other visual concerns is possible that it can negatively affect an opportunity to drive the car or potentially dangerous mechanisms. In this case it is necessary to wait some time before recovery of sight before driving the car or to use mechanisms.
The overdose
Is not present data on overdose by drug at topical ophthalmologic administration. It is improbable that use more than one drop in one eye can result in undesirable side effects. Practically there is no risk of development of side effects because of accidental ingestion of drug.
A form of release and packing
On 5 ml of drug in the bottle from polyethylene of low density corked by the dosing tip and a white cap from polypropylene.
On 1 bottle together with the instruction for medical use in the state and Russian languages place in a cardboard pack.
To Store storage conditions at a temperature not above 30 °C.
To store out of children’s reach.
2 years
not to apply a period of storage after an expiration date.
The use period after the first opening of a bottle – 4 weeks.
Prescription status
According to the prescription
the Producer Alkon-Kuvrer
of B-2870 of Puurs, Belgium
Additional information
| Ingredient |
|---|















Reviews
There are no reviews yet.