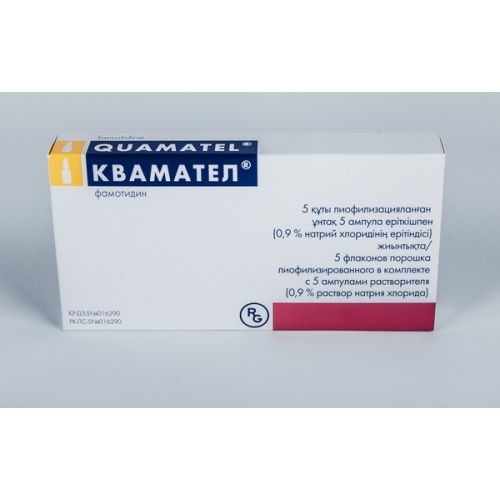
Kvamatel 5’s 20 mg lyophilized powder for injection
$28.60
Description
The instruction for medical use
of Kvamatel® medicine
the Trade name
of Kvamatel®
the International unlicensed
name Famotidine Dosage Form Powder lyophilized for preparation of solution for injections complete with solvent (0.9% chloride sodium solution)
Structure
One bottle with lyophilisate contains
active agent – famotidine of 20 mg,
excipients: aspartic acid, Mannitolum
solvent: sodium chloride, water for injections
the Description
the Lyophilized powder of white or almost white color
Pharmacotherapeutic group
Antiulcerous drugs and drugs for treatment of a gastroesophageal reflux (GORD). Blockers of H2-histamine receptors. Famotidine.
The ATX A02B A03 code
the Pharmacological
Pharmacokinetics Kinetics properties of famotidine has linear character.
Absorption
of Kvamatel® for injections it is intended only for intravenous administration.
Distribution
Linking with proteins of plasma is significant rather poorly: 15-20%.
Elimination half-life: 2.3 – 3.5 hours. At patients with a heavy renal failure time of semi-removal of famotidine can exceed 20 hours.
Biotransformation
Metabolism of drug occurs in a liver. The only metabolite found in the person is sulphoxide.
Removal
Famotidine is removed by kidneys (65 – 70%) and by metabolism (30 – 35%). The renal clearance is 250 – 450 ml/min. that indicates some extent of canalicular excretion. 65 – 70% of intravenously entered dose are found in urine in not changed look. A small part of the entered dose can be excreted in the form of sulphoxide.
The pharmacodynamics
Famotidine is a powerful competitive inhibitor of H2 – histamine receptors. The main clinically significant pharmacological effect of famotidine is the inhibition of gastric secretion. Famotidine reduces both concentration of acid, and volume of gastric secretion while changes of pepsinia are proportional to the cosecreted volume.
At healthy volunteers and patients with hypersecretion famotidine inhibits basal and night secretion of a stomach and also the secretion stimulated by administration of Pentagastrinum, beta ashes, caffeine, insulin and a physiological vagal reflex.
Secretion inhibition duration when using doses of 20 mg and 40 mg is from 10 to 12 hours.
Single oral administration of doses of 20 mg and 40 mg provides inhibition of basal and night secretion of acid in the evening.
Famotidine practically does not affect gastrin level in serum on an empty stomach or after meal.
Famotidine does not affect gastric emptying, exocrine function of a pancreas, a blood stream in a liver and a portal system.
Famotidine does not affect the fermental system of P450 cytochrome in a liver.
Anti-androgenic influence of drug is noted. Level of serumal hormones after treatment by famotidine did not change.
Indications
– a duodenum ulcer
– stomach ulcer without malignancy
– a gastroezofagealnorefluxny disease
– other states which are followed by hypersecretion (for example, Zollingera-Ellison’s syndrome)
– prevention of aspiration of acid gastric contents (Mendelssohn’s syndrome) when carrying out the general anesthesia
the Route of administration and doses
of Kvamatel® for injections is intended only for intravenous administration, recommended for use in stationary conditions, at patients who have no opportunity to take the drug inside.
Drug can be used until performing oral therapy does not become possible.
The recommended dose makes 20 mg intravenously (in / c) twice a day (each 12 hours).
At Zollingera-Ellison’s syndrome
the Initial dose makes 20 mg in in each 6 hours. After that the dosage depends on the volume of secretion and a clinical condition of the patient.
At the general anesthesia for prevention of aspiration of acid gastric contents
of 20 mg in at morning in day of operation or, at least, in 2 hours prior to operation.
The initial dose for intravenous administration cannot exceed 20 mg. For carrying out an intravenous injection contents of a bottle it is necessary to dissolve 0.9% of solution of sodium of chloride (solvent ampoule) in 5 – 10 ml, and then to enter slowly (within not less than 2 minutes). Divorced solution is stable within 24 hours at a temperature not over 25 of 0C.
At drug infusion, solution should be entered for 15 – 30 minutes. Solutions should be prepared just before introduction. It is possible to use only transparent colourless solutions.
In a renal failure
As famotidine is excreted mainly by kidneys, at patients with a heavy renal failure it is necessary to observe precautionary measures.
If the clearance of creatinine is less than 30 ml/min., and serumal concentration of creatinine exceeds 3 mg / 100 ml, then the daily dose needs to be lowered to 20 mg or to increase intervals between introductions till 36 – 48 o’clock.
Use for children
Safety and efficiency of drug at children are not established.
Use for elderly patients
Dose adjustment depending on age is not required.
Side effects
Seldom (1/10000 – 1/1000)
– a headache, dizziness
– diarrhea, a constipation
Very seldom (& lt, 1/10000)
– an agranulocytosis, a leukopenia, a pancytopenia, thrombocytopenia
– an anaphylaxis, a Quincke’s disease
– anorexia, sensations of discomfort in a stomach, nausea, vomiting, dryness in a mouth
– a depression, hallucinations, excitement, alarm, confusion of consciousness
– arrhythmia, atrioventricular block
– a bronchospasm
– cholestatic jaundice
– an acne, an alopecia, xeroderma, a toxic epidermal necrolysis, a small tortoiseshell, an itching
– an arthralgia, muscular spasms
– a gynecomastia (at the termination of treatment has reversible character)
– fatigue, slight fever
–
Does not know a deviation of level of liver enzymes (there is no opportunity to determine on the basis of the available data)
– a ring in
Contraindication ears
– hypersensitivity to any component of drug
– children’s and teenage age up to 18 years
– pregnancy and the period of a lactation (due to the lack of experience of use)
Medicinal interactions
Famotidine does not affect the fermental system of P450 cytochrome in a liver. Due to the increase rn secretion of a stomach famotidine can cause decrease in absorption of a ketokonazol at simultaneous use of these drugs.
Special instructions
before therapy by famotidine or if there is no such opportunity, before transition to oral treatment it is necessary to exclude existence of a malignant new growth of a stomach.
In a liver failure of Kvamatel® it is necessary to use with care in a reduced dose.
As in case of use of antagonists of H2 receptors the cross-responsiveness, use of Kvamatela® for the patients having in the anamnesis hypersensitivity to other antagonists of H2 receptors was described demands care.
Pregnancy and the period of a lactation
Adequate or well controlled researches at pregnant women were not conducted. Квамател® it is not recommended to apply during pregnancy.
Famotidine cosecretes with breast milk at the person, in this regard breastfeeding during use of Kvamatela® should be stopped.
Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
Data on any influence of famotidine on ability to run vehicles and to work with mechanisms are absent.
Overdose
At patients with a syndrome of pathological hypersecretion doses up to 800 mg a day throughout the period over one year were used that was not followed by emergence of the serious undesirable phenomena.
Treatment: symptomatic and maintenance therapy, monitoring of a condition of the patient.
The form of release and packing
place Drug in the bottles from transparent glass corked by a rubber bung and closed by an aluminum cap.
On 5 ml of solvent in ampoules from colourless glass with a tag for a break.
On 5 bottles with drug and 5 ampoules with solvent place in plastic blister strip packaging.
On 1 blister strip packaging together with the instruction for medical use in the state and Russian languages place in a pack from cardboard.
To Store storage conditions in original packing, in the place protected from light, at a temperature not over 25 of 0C.
To store out of children’s reach!
2 years
not to use a period of storage after the expiry date specified on packing.
Prescription status
According to the prescription
Only for stationary use (as an exception, according to the special order it is possible to apply in clinics or out of permanently)
the Name and the country
of the JSC Gideon Richter manufacturing organization,
1103 Budapest, Dyomryoi St., 19-21, Hungary
the Name and the country of the owner of the registration certificate
of JSC Gideon Richter, Hungary
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products:
Representative office of JSC Gideon Richter in RK
E-mail: info@richter.kz
Phone number: 8-(727) 258-26-22, 8-(727) 258-26-23
To develop
Additional information
| Ingredient |
|---|



















Reviews
There are no reviews yet.