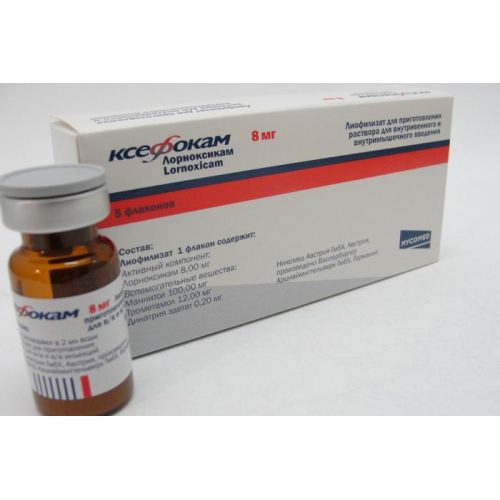
Ksefokam 5’s 8 mg lyophilized powder for injection
$37.00
Description
The instruction for medical use
of KSEFOKAM medicine
the Trade name
Ksefokam
Mezhdunarodnoye the unlicensed
name Lornoksikam Lekarstvennaya a form
Lyophilisate for preparation of solution for intravenous and intramuscular administration, 8 mg
Structure
1 bottle contains
active agent – to lornoksika, 8 mg
excipients: Mannitolum, trometamol, dinatrium edetat
Description
Powder of the yellow
Prepared Solution color: yellow transparent liquid without visible mechanical inclusions
Pharmacotherapeutic group
Anti-inflammatory and antirheumatic drugs. Non-steroidal anti-inflammatory drugs. Oksikama. Lornoksikam
the ATX M01AC05 Code
the Pharmacological
Ksefokama Pharmacokinetics Lyophilisate properties of 8 mg is intended as for intravenous and intramuscular administration. After an intramuscular injection the peak concentration in plasma are reached approximately in 0.4 hours. The absolute bioavailability (counting on AUC) is 97%.
Lornoksikam is provided in blood plasma in not changed look, or in the form of its hydroxylated metabolites. Linking of proteins of plasma with Lornoksikam makes 99% and does not depend on concentration.
Lornoksikam is substantially metabolized in a liver by hydroxylation in inactive to 5-hydroksilornoksika. CYP2C9 enzyme participates in metabolism of a lornoksikam. For this enzyme the patients are subdivided into slow and extensive metabolizator. In a type of this genetic polymorphism the level of a lornoksikam can increase considerably at slow metabolizator. Hydroxylated metabolites do not show pharmacological activity. Lornoksikam is metabolized completely: about 2/3 it is removed through a liver and 1/3 through kidneys in the form of inactive substances. Elimination half-life of initial connection makes from 3 to 4 hours.
The pharmacodynamics
Lornoksikam represents non-steroidal anti-inflammatory drug (NPVP) with the significant analgetic action. Oppression of synthesis of prostaglandins owing to the balanced oppression of activity of isoenzymes of cyclooxygenase-1 and cyclooxygenase-2 is the cornerstone of the mechanism of action of a lornoksikam. Besides, to lornoksika inhibits release of oxygen radicals from the activated leukocytes.
Lornoksikam does not possess opiatopodobny action on the central nervous system and therefore breath does not oppress and also does not cause constipations and miotic effect. There are also no bases to assume possibility at patients of medicinal dependence to a lornoksikam.
Indications – short-term treatment of a pain syndrome of various etiology.
The route of administration and doses
At impossibility of administration of drug inside in the tableted form apply intravenously or intramusculary.
Solution for injections is prepared just before use by dissolution of contents of a bottle (8 mg of the powder Ksefokam) water for injections (2 ml).
After solution preparation the needle is replaced. Intramuscular injections do by a long needle.
Duration of intravenous administration of solution has to be not less than 15 seconds, intramuscular – not less than 5 seconds.
The initial dose can make 8 or 16 mg. At insufficient analgesic effect of a dose it is possible to enter the same dose into 8 mg in addition.
Maintenance therapy: on 8 mg two times a day.
The maximum daily dose should not be more than 16 mg.
At intra articulate introduction of Ksefokam in arthritises the recommended dose makes 8 mg. Duration and frequency rate of therapy depends on disease severity and is established individually by the doctor.
Side effects
Often:
a headache, dizziness
an abdominal pain, dyspepsia, nausea, vomiting, diarrhea
Infrequently
– anorexia, change of weight
– insomnia, a depression
– conjunctivitis
– dizziness, sonitus
– heartbeat disturbance, tachycardia, heart failure hypostases (peripheral and generalized)
– rhinitis
– a constipation, a meteorism, an eructation, dryness in a mouth, ulcers in an oral cavity
– increase in level of hepatic transaminases, an abnormal liver function
– rash, an itching, erythematic rash, a small tortoiseshell and a Quincke’s edema, an alopecia, perspiration, hyperaemia
– an arthralgia
– an indisposition, a face edema
Seldom:
– a melena, vomiting with blood, an esophagitis, gastritis, a gastroesophageal reflux, a dysphagy
– erosive cankers of a mucous membrane of a stomach and intestines, including with perforation and bleeding
– aphthous stomatitis, a glossitis, pharyngitis, an asthma, cough, a bronchospasm
– anemia, thrombocytopenia, a leukopenia, increase in a bleeding time
– hypersensitivity, anaphylactoid reaction and an anaphylaxis
– confusion of consciousness, nervousness, excitement, drowsiness, paresthesia, a tremor, migraine
– disturbance of taste
– a disorder of vision
– a hypertension, inflows, bleeding, hematomas
– an ostealgia, muscular spasms, myalgia
– a nocturia, urination disturbance, increase in level of creatinine and urea nitrogen in blood serum
– an asthenia
– dermatitis and eczema, a purpura
Very seldom
– a neutropenia, an agranulocytosis, aplastic and hemolytic anemia
– the aseptic meningitis arising mainly at patients with a system lupus erythematosus or the mixed diseases of connective tissue
– a liver failure, hepatitis, jaundice, a cholestasia
– an edematous syndrome, bullous reaction, Stephens-Johnson’s syndrome, the Lyell’s disease
– an acute renal failure, interstitial nephrite, a nephrotic syndrome.
Contraindications
– hypersensitivity to a lornoksikam, acetylsalicylic acid or other NPVP
– instructions in the anamnesis on bronchial asthma, a small tortoiseshell and rhinitis
– thrombocytopenia
– heavy heart failure
– gastrointestinal, cerebrovascular and other bleedings or suspicion of bleeding, fibrillation disturbance
– a peptic ulcer of a stomach and duodenum in an aggravation phase, in the anamnesis of bleeding from a GIT, NPVP connected with reception
– the profound liver failure
– the profound renal failure (level of serumal creatinine & gt, 700 µmol/l)
– children’s and youthful age up to 18 years
pregnancy and the period of a lactation.
Medicinal interactions
At simultaneous use of the drug Ksefokam and:
– Cimetidinum concentration of a lornoksikam in plasma of blood increases, interactions with ranitidine and antiacid drugs it is not revealed,
– anticoagulants or inhibitors of aggregation of thrombocytes – increase in a bleeding time and increase in risk of developing bleeding (control of the international normalized MNO relation is necessary) is possible,
– – adrenoblockers and APF inhibitors can reduce their hypotensive effect,
– diuretics the diuretic effect and hypotensive action decreases
– digoxin reduces renal clearance of digoxin,
– hinolonovy antibiotics risk of development of a convulsive syndrome increases,
– other non-steroidal anti-inflammatory drugs and glucocorticoids – the risk of a GIT of bleedings increases,
– a methotrexate concentration of a methotrexate in serum increases,
– selective serotonin reuptake inhibitors (for example, a tsitaloprama, fluoxetine, a paroksetin, sertraline) the risk of a GIT of bleedings increases,
– salts of lithium can cause increase in the maximum concentration of lithium in plasma and by that strengthening of the known side effects of lithium,
– cyclosporine increases nephrotoxicity of cyclosporine,
– derivatives of sulphonylurea is possible strengthening of hypoglycemic effect of the last,
– the pemetrekseda can reduce clearance of a pemetreksed.
– alcohol, corticotropin, drugs of potassium increase in risk of by-effects from a GIT is possible,
– a tsefamandola, a tsefoperazona, a tsefotetana, valprovy acid the risk of bleeding increases.
Special instructions
Renal failure:
At patients with a renal failure (level of serumal creatinine of 150-300 µmol/l), in heart failure, in the abnormal liver function and other states causing decrease in volume of the circulating blood and a renal blood-groove carry out continuous clinical monitoring, control of function of kidneys (for example, on the level of serumal creatinine) and a liver.
Long-term treatment (more than 3 months):
It is regularly recommended to carry out the assessment of a condition of blood (hemoglobin), functions of kidneys (creatinine) and liver enzymes.
To patients 65 years, or with body weight less than 50 kg are more senior, or transferred extensive surgeries it is recommended to enter smaller doses of Ksefokam. The total daily dose should not exceed 8 mg.
At prolonged use of the drug Ksefokam it is necessary to control a pattern of peripheral blood and also indicators of function of a liver and kidneys.
Gastrointestinal ulcers and bleedings in the anamnesis.
Carrying out clinical observation is recommended. In a round ulcer of a stomach and a peptic ulcer of a duodenum the therapy by Ksefokam should be carried out against the background of intake of inhibitors of a proton pomp (pantoprazol).
It is not necessary to apply along with other NPVP.
Patients it is necessary to be careful with existence in the anamnesis of hypertensia and/or heart failure, drug can cause a delay of water and sodium, peripheral hypostases and arterial hypertension.
At emergence of signs of damage of a liver (skin itching, yellowing of integuments, nausea, vomiting, an abdominal pain, urine darkening, increase in level of hepatic transaminases) it is necessary to stop administration of drug and to see the attending physician.
As well as other oksikama, Ksefokam oppresses aggregation of thrombocytes and therefore can increase a bleeding time. At use of this drug it is necessary to watch attentively a condition of the patients needing absolutely normal functioning of a system of fibrillation (for example, patients, to whom is necessary surgical intervention), having disturbances of a system of fibrillation or receiving the medicines oppressing coagulation (including heparin in low doses) in due time to find symptoms of bleeding.
Patients with genetic intolerance of a galactose, deficiency
of lactase or glyukozo-galaktozny malabsorption it is not necessary to take the drug Ksefokam.
Use of drug can negatively affect female fertility and is not recommended to the women planning pregnancy.
Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms.
It is necessary to refrain from control of the vehicles and other mechanisms requiring special attention because of the probability of dizzinesses and drowsiness.
Overdose
Symptoms: nausea and vomiting, cerebral symptoms (the dizziness, an ataxy passing into a coma and spasms). Changes of function of a liver and kidneys, disturbances of blood clotting are possible.
Treatment: it is necessary to stop drug intake, symptomatic treatment. Thanks to short elimination half-life, lornoksika it is quickly brought out of an organism. Lornoksikam is not exposed to dialysis. So far specific antidote is not known. Intake of activated carbon right after administration of drug Ksefokam can promote decrease in absorption of this drug. For elimination of the gastrointestinal disturbances caused by the drug Ksefokam it is possible to use an analog of prostaglandin or ranitidine.
The form of release and packing
On 8 mg of active agent place in the bottle from brown glass corked by a rubber bung and which is pressed out by an aluminum cap.
On 5 bottles together with the instruction for medical use in the state and Russian languages put in a cardboard box.
or
On 8 mg of active agent place in the bottle from brown glass corked by a rubber bung and which is pressed out by an aluminum cap.
On 5 bottles in a plastic pallet together with the instruction for medical use in the state and Russian languages put in a cardboard box.
To Store storage conditions in the place protected from light at
a temperature not higher than 25 Store OS out of children’s reach!
The period of storage
the Prepared solution needs to be used 3 years within 24 hours!
Not to use drug after expiry date
Prescription status
According to the prescription
the Name and the country
of the manufacturing organization Wasserburger Artsnaymittelverk GmbH, Wasserburg, Germany
the Name and the country of the owner of the registration certificate
Takeda Austria GmbH, Austria
the Name and the country of the organization packer
Takeda Austria GmbH, Austria
The address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods)
Representative office
Takeda Osteuropa Holding GmbH (Austria) in
Kazakhstan Almaty, Shashkin St. 44 Phone number
(727) 2444004 Fax number (727) 2444005
To develop the e-mail address of DSO-KZ@takeda.com
Additional information
| Ingredient |
|---|













Reviews
There are no reviews yet.