Description
The instruction for medical use
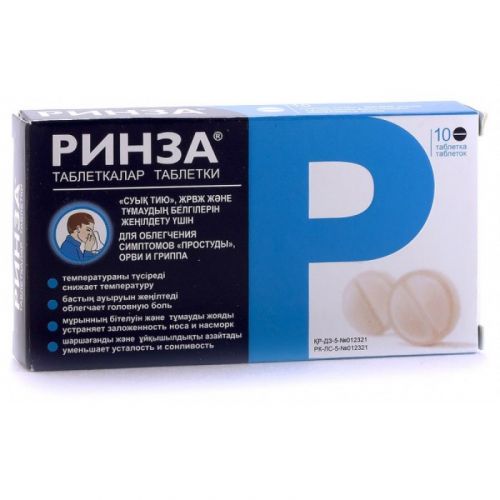
of RINZA® medicine
the Trade name
of Rinza®
the International unlicensed name
Is not present
the Dosage form
of the Tablet Structure One Tablet contains
active agents: paracetamol of 500 mg
caffeine of anhydrous 30 mg
of Phenylephrinum hydrochloride of 10 mg
of chlorpheniramine maleate of 2 mg,
excipients: silicon dioxide colloidal anhydrous, dye crimson 4R (E124), starch corn, povidone (K-30), sodium methylparahydroxybenzoate, magnesium the stearate, talc purified of sodium glikolit starch, the water purified.
The description
Round flat tablets of pink color without cover with dark pink and white impregnations with slanted edges and dividing risky on one party.
Pharmacotherapeutic group
Analgetics. Other analgetics-antipyretics. Anilides. Paracetamol in a combination with other
drugs ATX N02BE51 Code Pharmacological Pharmacokinetics Later Properties of intake paracetamol is quickly absorbed from the digestive tract (DT), mainly in a small intestine. After single dose in a dose of 500 mg the maximum concentration in blood plasma (Cmax) is reached in 10-60 min. and makes about 16 mkg/ml, then gradually decreases and in 6 h makes 11-12 mkg/ml. Linking with blood proteins makes less than 10%. It is removed with urine mainly in the form of glyukuronidny and sulphatic conjugates.
Caffeine is metabolized in a liver (97%) in 1.7 methylxanthine, 1.7 dimethylmethylxanthine and 1.3-dimethyl uric acid which are removed with urine.
After intake Phenylephrinum is badly soaked up from a GIT. It is metabolized with the participation of MAO in a wall of intestines and at the first passing through a liver. Bioavailability of Phenylephrinum low.
Chlorfeniramin well gets into different fabrics and through a blood-brain barrier. It is metabolized in a liver by methylation. It is removed in the form of metabolites and in not changed look by kidneys and a GIT.
The pharmacodynamics
of Rinza® possesses analgeziruyushchy, febrifugal and antiallergic the actions caused by the active agents which are a part of drug.
Paracetamol has analgeziruyushchy and febrifugal effect: reduces the pain syndrome which is observed at catarrhal states – a sore throat, a headache, muscular and articulate pain, reduces high temperature. The mechanism of action is connected with suppression of synthesis of prostaglandins, mainly in the center of thermal control in a hypothalamus.
Caffeine has the stimulating influence on the central nervous system (CNS) that leads to increase in intellectual and physical effeciency.
Phenylephrinum a hydrochloride – α1-адреномиметик. Has vasoconstrictive effect, reduces swelled also hyperaemia of mucous membranes of upper parts of airways and adnexal bosoms of a nose.
Chlorpheniramine a maleate – a blocker histamine H1 receptors, have antiallergic effect, reduce puffiness and hyperaemia of mucous membranes of a nasal cavity, nasopharynx and adnexal bosoms of a nose, eliminate an itching of eyes and a nose, reduce exudative manifestations.
Indications
Symptomatic treatment:
– flu
– a SARS (feverish syndrome, a pain syndrome, a rhinorrhea)
– allergic rhinitis
– other catarrhal diseases which are followed by rhinitis, congestion of a nose, a headache, fever, a fever, joint, muscles pain.
The route of administration and doses
appoint Drug inside in 1-2 h after meal.
To adults and children 12 years are more senior each 4-6 h appoint on 1 tablet. The maximum single dose – 1 tablet, the maximum daily dose – 4 tablets. A course of treatment – no more than 5 days.
Side effects
Serious skin reactions:
Very seldom:
– Sharp Generalized Exanthematous Pustulez (SGEP). Acute condition with development of pustulous rashes. It is characterized by the fever and a diffusion erythema which is followed by burning and an itching. There can be edema of face, hands and mucous
– the Stephens’s Syndrome — Johnson (SSJ) (malignant exudative erythema). Severe form of a mnogoformny erythema at which there are bubbles on a mucous membrane of an oral cavity, a throat, eyes, genitals, other sites of skin and mucous membranes
– the Toxic epidermal necrolysis (TEN, a Lyell’s disease). The syndrome is a consequence of extensive apoptosis of keratinotsit that leads to amotio of extensive sites of skin in places of dermoepidermalny connection. The affected skin has an appearance scalded by boiled water.
If you noticed one of the side effects described above, it is necessary to stop administration of drug and to see immediately a doctor!
Allergic reactions: skin rash, itching, small tortoiseshell, Quincke’s disease.
From nervous system: dizziness, backfilling disturbance, hyperexcitability.
From a cardiovascular system: increase in arterial blood pressure, tachycardia.
From digestive system: nausea, vomiting, pain in epigastric area, dryness of a mucous membrane of an oral cavity, hepatotoxic action.
From sense bodys: mydriasis, accommodation paresis, increase in intraocular pressure.
From bodies of a hemopoiesis: anemia, thrombocytopenia, agranulocytosis, hemolytic anemia, aplastic anemia, methemoglobinemia, pancytopenia.
From an urinary system: ischuria, nephrotoxicity (renal colic, glucosuria, interstitial nephrite, papillary necrosis).
Other: bronchospasm.
Contraindications
– hypersensitivity to the paracetamol and other components which are a part of drug
– intake of other drugs containing the substances which are a part of Rinzy®
– the profound atherosclerosis of coronary arteries
– arterial hypertension (heavy course)
– diabetes (heavy course)
– a concomitant use of tricyclic antidepressants, inhibitors of a monoaminooxidase, beta blockers
– pregnancy, the lactation period
– children’s age up to 12 years
– alcoholism
With care – arterial hypertension, a thyrotoxicosis, a pheochromocytoma, diabetes, bronchial asthma, a chronic obstructive pulmonary disease, deficit glyukozo-6-fosfatdegidrogenazy, blood diseases, inborn hyperbilirubinemias (Gilbert’s syndromes,
the Cudgel Johnson and the Rotor), a liver and/or renal failure, closed-angle glaucoma, a prostate hyperplasia.
Medicinal interactions
Enhances effects of inhibitors of a monoaminooxidase, sedative drugs, ethanol.
Antidepressants, protivoparkinsonichesky means, antipsychotic means, fenotiazinovy derivatives – increase risk of development of an ischuria, dryness in a mouth, constipations. Glucocorticosteroids increase risk of developing glaucoma. Paracetamol reduces efficiency of diuretic drugs. Chlorpheniramine along with monoaminooxidase inhibitors, furasolidone can lead to hypertensive crisis, excitement, a hyper pyrexia. Tricyclic antidepressants strengthen adrenomimetichesky effect of Phenylephrinum, co-administration of a halothane increases risk of developing ventricular arrhythmia. Reduces hypotensive action of a guanetidin who, in turn, enhances alpha adrenostimuliruyushchuyu activity of Phenylephrinum.
At co-administration of Rinzy® with barbiturates, dipheninum, carbamazepine, rifampicin and other inductors of microsomal enzymes of a liver the risk of development of hepatotoxic action of paracetamol increases.
Special instructions
during treatment it is necessary to refrain from alcohol intake, sleeping and anxiolytic (tranquilizers) medicines. Not to accept together with other medicines containing paracetamol. If symptoms of a disease do not pass within 3-5 days, to see a doctor.
The feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
Should refrain from driving by motor transport and occupations with potentially dangerous mechanisms.
Overdose
in case of overdose it is necessary to see a doctor immediately. Fast delivery of health care is crucial even if you do not observe any signs or symptoms.
Symptoms (are caused, generally by paracetamol), are shown after reception over 7.5 – 10 g: during the first 24 h after reception – pallor
of integuments, nausea, vomiting, anorexia, abdominal pain, increase in a prothrombin time, glucose metabolism disturbance. Symptoms of an abnormal liver function can appear in 12 – 48 h after overdose: increase in activity of ‘hepatic’ transaminases, gepatonekroz. In hard cases – a liver failure with the progressing encephalopathy, a coma. Seldom liver failure develops immediately and can be complicated by a renal failure (tubular necrosis).
Treatment: gastric lavage, prescribing of activated carbon in the first 6 h after overdose, introduction of donators of SH-group and predecessors of synthesis of glutathione – methionine in 8 – 9 h after overdose and Acetylcysteinum in 12 h. Need for holding additional therapeutic actions (further administration of methionine and Acetylcysteinum) is defined by concentration of paracetamol in blood and also time which passed after its reception. Symptomatic therapy is shown.
The form of release and packing
On 10 tablets place in blister strip packaging from printing aluminum foil and a film polyvinylchloride.
On 1 planimetric packing together with the instruction for medical use in the state and Russian languages place in a cardboard box.
To Store storage conditions in the dry, protected from light place, at a temperature not above 25 °C.
To store out of children’s reach!
3 years
not to apply a period of storage until expiry date.
Prescription status
Without prescription
Unique Pharmaceutical Laboratories Producer (department of firm J.B. Kemikals and Pharmasyyutikals Ltd.)
Nilam Senter, a wing of B, the 4th floor, Hind Saykl Road, Vorley, Mumbai – 400,030, India
the Owner of the registration certificate
of LLC Johnson & Johnson,
Russia 121614, Russia, Moscow, Krylatskaya St., 17, building 2
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods)
LLC Johnson & Johnson Branch in
Republic of Kazakhstan 050040, Republic of Kazakhstan, Almaty, Timiryazev St., 42, pavilion 23-A
ph./fax: +7 (727) 356-88-11, +7 (727) 356-88-19
e-mail:
To Develop safetyru@its.jnj.com














Reviews
There are no reviews yet.