Description
The instruction for medical use
of PANCREATINUM medicine
the Trade name
Pancreatinum
the International unlicensed name
Is not present
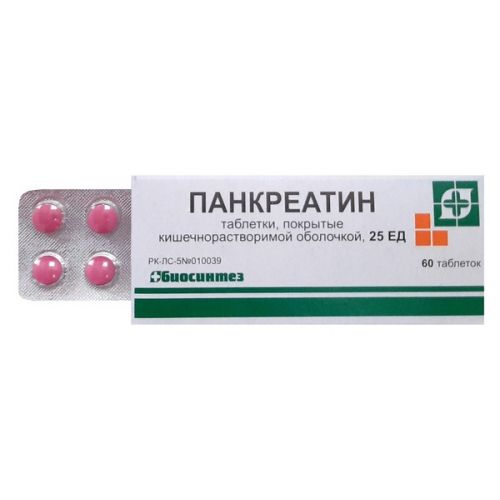
the Dosage form
of the Tablet, covered with a kishechnorastvorimy cover, 25 PIECES
Structure
One tablet contains
active agent – Pancreatinum of 0.1 g,
excipients
a kernel: lactose (sugar milk), gelatin, potato starch, calcium stearate,
cover: tsellatsefat (atsetilftaliltsellyuloza), the titan dioxide (the titan dioxide) E171, liquid paraffin (liquid paraffin), polysorbate (twin-80), an azoruby (dye acid red 2C)
the Description
of the Tablet of a biconvex form, coated pink or dark pink color, with a specific smell. On cross section two layers are visible, in an inside layer impregnations
Pharmacotherapeutic group
Drugs are allowed, digestant (including fermental drugs)
the Digestive fermental
drugs ATX A09AA02 Code Pharmacological Pharmacokinetics Pancreatic Enzymes Properties are released from a dosage form in the alkaline environment of a small intestine since are protected from effect of gastric juice by a cover.
The maximum enzymatic activity of drug is noted in 30-45 min. after oral administration.
The pharmacodynamics
Digestive fermental means, fills shortage of enzymes of a pancreas, has proteolytic, amylolytic and lipolytic effect. The pancreatic enzymes (lipase, alpha amylase, trypsin, chymotrypsin) which are a part promote the proteolysis to amino acids, fats – to glycerin and fatty acids, starch – to dextrins and monosaccharides, improve a functional condition of digestive tract, normalize digestion processes.
Indications
– replacement therapy at vneshnesekretorny insufficiency of a pancreas: chronic pancreatitis, pancreatectomy, a state after radiation, dyspepsia, a mucoviscidosis
– a meteorism, diarrhea of non-infectious genesis
– disturbance of digestion of food (a state after a resection of a stomach and a small intestine)
– for improvement of digestion of food at persons with normal function of digestive tract in case of errors in food (consumption of greasy food, a large number of food, irregular food) and at disturbances of chewing function, inactive lifestyle, a long immobilization
– Remkheld’s syndrome (gastrocardial syndrome)
– preparation to radiological and to ultrasound examination of abdominal organs
the Route of administration and doses
take the Drug inside in time or after a meal, without chewing and washing down with not alkaline liquid (water, fruit juice).
The dose of drug is established individually depending on age and degree of insufficiency of a pancreas. One tablet contains: proteases – 25 PIECES, amylases – 1700 PIECES, lipases – 150 PIECES.
The adult usually – on 2-4 tablets of 3-6 times a day. The maximum daily dose – 16 tablets. Duration of treatment is defined by the attending physician.
Side effects
– allergic reactions
seldom:
– diarrhea, a constipation, sensation of discomfort in a stomach, nausea (relationship of cause and effect of development of these reactions is not established with action of Pancreatinum since the specified phenomena belong to symptoms of vneshnesekretorny insufficiency of a pancreas)
– a hyper uricosuria, a hyperuricemia (at prolonged use in high doses)
– development of strictures (fibrous colonopathy) in ileocecal department of the ascending colon in a mucoviscidosis in case of exceeding a necessary dose of Pancreatinum
of the Contraindication
– hereditary intolerance of fructose, deficiency of Lapp enzyme – lactase, glucose malabsorption – galactoses
– hypersensitivity to drug components
– acute pancreatitis, exacerbation of chronic pancreatitis
– pregnancy and the period of a lactation
– children’s and teenage age up to 18 years
Medicinal interactions
At use of Pancreatinum is possible decrease in absorption of iron and folic acid. Simultaneous use of the antiacid means containing calcium a carbonate and/or magnesium hydroxide can lead to decrease in efficiency of Pancreatinum.
Special instructions
In a mucoviscidosis the use of Pancreatinum in high doses owing to increase in risk of development of strictures (fibrous colonopathy) is not recommended.
The dose has to be adequate to amount of enzymes which is necessary for absorption of fats taking into account quality and amount of the consumed food.
Pregnancy and a lactation
As drug contains the dye (azoruby) forbidden for children it it is impossible to apply pregnant and feeding.
The feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
does not influence.
Overdose
Symptoms: hyper uricosuria, hyperuricemia, zaporoshok
Treatment: drug withdrawal, symptomatic therapy.
The form of release and packing
On 60 tablets place in banks polymeric the BP type.
On 10 tablets place in blister strip packaging from a film of the polyvinylchloride and printing aluminum foil varnished or from paper with a polyethylene covering.
Each can or 6 blister strip packagings together with the instruction for use in the state and Russian languages are placed in a pack from cardboard.
To Store storage conditions in the dry place at a temperature not higher than 20 wasps.
To store out of children’s reach!
Period of storage
2 years.
Not to apply after an expiration date.
Prescription status
Without prescription.
JSC Biosintez producer,
Russian Federation 440033, Penza, Druzhby St., 4, ph. / fax (8412) 57-72-49
e-mail: certificate@biosintez.com
the Owner of the registration certificate
of JSC Biosintez, the Russian Federation
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods)
of JSC Biosintez,
Russian Federation 440033, Penza, Druzhby St., 4, ph. / fax (8412) 57-72-49
e-mail: certificate@biosintez.com
Additional information
| Ingredient |
|---|




















Reviews
There are no reviews yet.