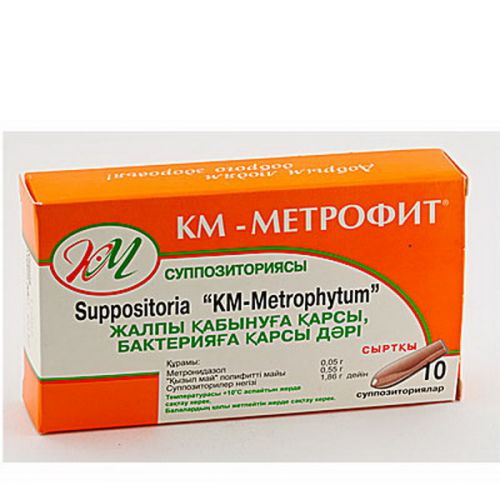
Metrofit 10s suppositories
$9.10
Description
Instruction for medical use
of KM medicine – Метрофит®
the Trade name
of KM – Metrofit®
the International unlicensed name
Is not present
the Dosage form
Suppositories
Structure
One suppository contains
active agents: metronidazole of 0.05 g, oil polifitovy Kyzyl May 0.55 g
excipients: wax of yellow 0.12 g, cocoa butter of 1.14 g.
The description
Suppositories from light-cream till cream color, uniform or with a hollow core in a section. At storage in the fridge the emergence of the impregnations due to crystallization of wax disappearing at the room temperature is possible.
The pharmacotherapeutic group
Other antiseptic agents and the antimicrobial
drugs Code of Automatic Telephone Exchange G01AF Pharmacological Metronidazole Properties has anti-protozoan and antibacterial properties.
When using the system absorption is minimum. Elimination half-life – 8-10 h. Communication with proteins of plasma small, & lt, 20%. It is metabolized, mainly, in a liver with education two not conjugated active oxidation metabolite (5% – 30% of activity). Removal is carried out, mainly, with urine, the metabolites removed with urine metronidazole and oxidized make about 35% – 65% of the absorbed dose.
Kyzyl May oil polifitovy possesses anti-inflammatory, regenerating, tonic, spasmolytic, antioxidant, anti-toxic, wound healing properties and has soft aperient effect.
KM – Metrofit® suppositories renders antibacterial, antiprotozoan, anti-inflammatory and reparative actions.
Indications
as a part of complex therapy:
– the colpitis, a cervicitis, an erosion
– cystitis, prostatitis
Enter the Route of administration and doses rektalno or vaginalno on 1 suppository 1-2 times a day, within 10-15 days
the Side effects
– are possible allergic reactions to the making
Contraindication drug components
– the increased individual sensitivity to drug components
– the lactation period
– the I trimester of pregnancy
Medicinal interactions
can lead Simultaneous use with Disulfiramum ment of various neurologic symptoms (it is not necessary to appoint metronidazole sick which accepted Disulfiramum during the last 2 weeks). (Warfarin) strengthens effect of indirect anticoagulants, it is not recommended to combine with not depolarizing muscle relaxants (a vekuroniya bromide). At a concomitant use with the drugs Li + concentration of the last in plasma can increase.
The special
instructions Use in pediatrics
the use for children is not recommended.
Pregnancy and a lactation
Drug can be used in therapeutic doses at pregnant women the II-III trimester taking into account coefficient advantage/risk.
Features influence of medicine on ability of control of vehicles and potentially dangerous mechanisms
does not influence.
The overdose
is not revealed
the Form of release and packing
On 5 or 10 suppositories in blister strip packaging from a film polyvinylchloride. Or two (on 5 suppositories) planimetric packings together with the instruction for use in the state and Russian languages place one (on 10 suppositories) in a pack from cardboard.
To Store storage conditions at a temperature not over 10 of 0C.
To store out of children’s reach!
A period of storage
3 years
not to use after the termination of an expiration date
the Address of the organization accepting in the territory of RK of a claim of consumers on quality of products
of the Kyzylmay personal computer Republic of Kazakhstan, Almaty, Ippodromnaya St., 6
of ph. 382-20-27, fax 382-19-20 E specified on packing Prescription status Without prescription PC maker Kyzylmay, Kazakhstan, Almaty, Ippodromnaya St., 6 the Name and the country of the owner of the registration certificate of the Kyzylmay personal computer, Kazakhstan – mail: reception@kyzylmay.com
Additional information
| Ingredient |
|---|


















Reviews
There are no reviews yet.