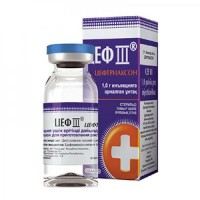
Gentamicin Sulfate 4% / 2 ml 10s solution for injection in ampoules
- $4.10
Out Of Stock
The instruction for medical use
of GENTAMYCINI SULFAS medicine
the Trade name
Gentamycini sulfas
the International unlicensed
name Gentamycin Dosage Form Solution for injections of 4%, 2 ml
Structure
of 2 ml of solution contain
active agent - Gentamycini sulfas (in terms of
gentamycin) - 80.0 mg,
excipients: sodium metabisulphite, dinatrium edetat, water for injections.
The description
the Transparent, colourless or slightly painted liquid
Pharmacotherapeutic group
Antibacterial drugs for system use. Aminoglikozidny antibacterial drugs. Other aminoglycosides. Gentamycin.
The ATX J01GB03 code
the Pharmacological
Pharmacokinetics Drug properties is administered intramusculary or intravenously. It is quickly and completely soaked up at intramuscular introduction. After intramuscular introduction of Gentamycini sulfas the peak plasma concentration are reached within 30 - 60 minutes, concentration can be defined in plasma from 6 to 12 hours. Linking of gentamycin with proteins of plasma low from 0 to 10%.
Gets into plasma, a lymph, fabrics (a liver, kidneys, lungs), a phlegm, pleural, synovial and peritoneal liquids and through a placental barrier. Concentration in cortical substance of kidneys can sometimes exceed by eight times usual levels in plasma. In bile low concentration are defined that confirms insignificant removal of gentamycin with bile. Badly gets through a blood-brain barrier. Concentration of gentamycin in cerebrospinal fluid low also depend on a dose, existence and expressiveness of inflammation of a meninx.
Gentamycin can collect in plasma and tissues of patients at introduction of high doses for a long time, especially in a renal failure or in malformations of kidneys. As gentamycin is distributed in extracellular liquid, peak plasma concentration at patients with large volumes of extracellular liquid can be lower, than usually.
Removal of gentamycin depends on post-natal age and clearance of creatinine. With increase in post-natal age and increase in a maturity of kidneys gentamycin is removed more quickly. Metabolic changes of gentamycin are minimum, removal happens by glomerular filtration. In several days of therapy the amount of the removed gentamycin is brought closer, but is not equal to quantity of the entered dose. A small part of the entered dose of gentamycin can remain in fabrics, in particular in kidneys. At some patients aminoglycosides were defined in insignificant quantity in urine in several weeks after the end of therapy. The renal clearance of gentamycin is similar to clearance of endogenous creatinine.
It is not metabolized. About 90% of gentamycin are removed in not changed look by kidneys by means of glomerular filtration.
At adults the elimination half-life at normal function of kidneys makes 2-3 hours, children aged from 1 week up to 6 months have 3 - 3.5 h, newborn and premature children with body weight have more than 2 kg – 5.5 h, with body weight less than 1.5 kg – 11.5 h, up to 2 kg - 8 h.
At the increased temperature and anemia the elimination half-life can be shortened. Dose adjustment usually is not required. At patients with heavy burns the elimination half-life can decrease considerably, causing decrease in plasma concentration in comparison with expected when dosing in body weight mg/kg.
With urine it is removed - 70–95% of gentamycin and a small amount - with bile.
At patients with a heavy renal failure the decrease in concentration of gentamycin in urine and its penetration into the struck parenchyma of kidneys is observed. It leads to decrease in removal of drug and increase in risk of nephrotoxic effect of aminoglycosides that it is necessary to take into account at treatment of patients with infections of urinary tract.
A pharmacodynamics
Gentamycini sulfas – an antibiotic of a broad spectrum of activity from group of aminoglycosides, antibacterial agent for topical and parenteral administration. Actively getting through a cellular membrane of bacteria, it is irreversible contacts 30S a subunit of bacterial ribosomes and by that activator protein synthesis oppresses. Gentamycin has bactericidal effect concerning many gram-positive and gram-negative microorganisms, including the so-called steady microbes resistant to other antibiotics.
It is active concerning a wide number of pathogenic bacteriums, including Escherichia coli, Proteus spp., Pseudomonas aeruginosa, Klebsiella spp., Enterobacter spp., Serratia spp., Citrobacter spp., Staphylococcus spp. (including the strains resistant to penicillin and Methicillinum).
Neisseria meningitides, Treponema pallidum, some strains of Streptococcus spp., anaerobic bacteria are resistant to gentamycin.
Does not affect mushrooms, viruses, the elementary.
Indications
- lower respiratory tract infections, bronchitis, pneumonia, pleurisy,
pleura empyemas
- infections of the central nervous system, including meningitis, as a part of
complex therapy
- the heavy complicated urinary tract infections, pyelonephritis, cystitis,
an urethritis, prostatitis
- a bacterial septicaemia, sepsis
- acute cholecystitis, a cholangitis, peritonitis
- purulent infections of skin and soft tissues, a burn infection
- infections of bones and joints
the Route of administration and doses
enter Gentamycini sulfas intramusculary or intravenously (by drop infusion). The mode of dosing is set individually taking into account the body weight of the patient.
Adult: 3 mg/kg/days, in heavy infections - 5 mg/kg/days (to about 80 mg there are each 8 hours).
The average duration of treatment – 6-7 days, as much as possible – 10 days.
The recommended doses are identical both for intramuscular, and for intravenous administration.
Solution for injections of Gentamycini sulfas cannot be mixed with other drugs, it is necessary to enter separately according to ways of introduction and the mode of dosing.
Maximum single dose: 160 mg (for patients with normal function of kidneys).
The maximum daily dose for all age groups: 5 mg/kg/days.
To children up to 3 years drug is appointed only according to vital indications in heavy infections
the Dosage for children of various age taking into account body weight.
Daily doses make:
to newborns and children of chest age – 2 – 5 mg/kg,
to children aged from 1 year up to 5 years – 1.5 - 3 mg/kg, 6 – 14 years – 3 mg/kg. The maximum daily dose for children of all age groups makes 5 mg/kg. The drug is administered by 2 – 3 times a day.
In a renal failure the correction of the mode of dosing is required. When carrying out a hemodialysis the recommended doses after its session for adults - 1-1.7 mg/kg (depending on weight of an infection), for children – 2 - 2.5 mg/kg.
At a heavy course of infections the purpose of smaller single doses with bigger frequency rate is recommended, decrease in size of a single dose has to be multiple to the relation calculated by the interval formula given above to the size of a normal interval between introductions (8 h). The dose has to be picked up so that Cmax did not exceed 12 mkg/ml (reduction of risk of development nefro-, from - and neurotoxicity).
In hypostases, ascites, obesity the dose is determined by 'ideal' or 'dry' body weight.
Whenever possible it is necessary to carry out control of gentamycin level in plasma.
Intravenous administration of the Dose same, as for intramuscular introduction. The usual volume of solvent (sterile normal saline solution or 5% glucose solution) is 100-200 ml for adults, for children the volume of solvent has to be reduced in proportion. Concentration of Gentamycin in solution should not exceed 1 mg/ml (0.1%). Solution is entered in the form of slow infusion within 1-2 hours. In the syringe Gentamycini sulfas cannot be mixed with any other medicine.
Side effects
- a headache, drowsiness, disturbance of neuromuscular conductivity,
muscular pains, spasms, vestibular disorders, decrease in hearing (in certain cases – irreversible deafness)
- nausea, vomiting, loss of appetite, decrease in body weight, the increased salivation
- increase in activity of hepatic transaminases, a hyperbilirubinemia
- anemia, a leukopenia, a granulocytopenia, thrombocytopenia
- an oliguria, a proteinuria, a microhematuria,
- a renal failure
- skin rash, an itching, a small tortoiseshell, fever, sometimes – a Quincke's edema
- a hypocalcemia, a gipokalemiya, a gipomagnemiya (at children)
In rare instances
- tubular necrosis
- an acute anaphylaxis
- morbidity in the place of intramuscular introduction
- phlebitis and thrombophlebitises at intravenous administration
- development of superinfection
of the Contraindication
- hypersensitivity to components of drug and other
antibiotics of group of aminoglycosides
- neuritis of an acoustical nerve
- a disease of a vestibular mechanism
- heavy renal failures, uraemia, an azotemia
- pregnancy and the period of a lactation
- a myasthenia, parkinsonism
- children's age up to 3 years
Medicinal interactions
It is necessary to take into account a possibility of development of neuromuscular blockade and paralysis of breath at any way of administration of aminoglycosides at the patients receiving the anesthetics or means causing neuromuscular blockade such as suktsinilkholin, tubocurarine, dekametony and also at patients to whom massive transfusions of citrated blood are carried out. At approach of neuromuscular blockade enter calcium salts.
Simultaneous or subsequent system or topical administration of other potentially neurotoxic or nephrotoxic drugs, such as Cisplatinum, Cefaloridinum, Kanamycinum, amikacin, Neomycinum, polimiksin-V, colistin, paromomitsin, streptomycin, Tobramycinum, Vancomycinum and Viomycinum is not recommended.
At simultaneous use of a hydrocortisone and indometacin the nephrotoxic effect of gentamycin can amplify.
It is not necessary to apply along with furosemide and Acidum etacrynicum because strengthening of ototoksichesky and nephrotoxic action is possible. Besides, at intravenous use of diuretics the change of concentration of an antibiotic in plasma and fabrics is possible that leads to strengthening of the toxic reactions caused by aminoglycosides.
At the patients with a heavy renal failure receiving at the same time karbenitsillin and gentamycin, decrease in elimination half-life of gentamycin from plasma was observed.
Pharmaceutical it is incompatible with a beta laktamnymi antibiotics, heparins, Amphotericinum.
Special instructions
At drug use whenever possible carry out monitoring of plasma concentration of gentamycin. Increase in an interval between introductions can be required. In view of the fact that concentration of creatinine of plasma have close correlation with elimination half-life of gentamycin, laboratory tests on creatinine can serve as the management for establishment of an interval between introductions.
At use of drug it is necessary to control functions of kidneys, acoustical and vestibular devices.
Gentamycin should be applied with care at patients with botulism, dehydration, parkinsonism, a hypocalcemia and also at patients of advanced age. The risk of ototoxicity increases in dehydration of an organism and at elderly people in this connection they have to use enough liquid. The patients having diseases of kidneys, a hearing loss, dizzinesses or sonitus are especially sensitive to gentamycin.
Introduction of all daily dose of Gentamycini sulfas in burns more than 20%, an endocarditis, sepsis is not recommended.
Drug is not recommended for treatment of not hospital pneumonia, both in out-patient, and in stationary conditions. Against the background of treatment the resistance of microorganisms can develop. In similar cases it is necessary to cancel drug and to appoint treatment on the basis of data of an antibiotikogramma. The bisulphite which is contained in sodium ampoules can cause development in sick allergic complications, especially in patients with the burdened allergic anamnesis.
Use in pediatrics
to Children up to 3 years only according to vital indications in heavy infections.
Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
Considering side effects of drug it is necessary to be careful at control of motor transport or potentially dangerous mechanisms.
Overdose
Symptoms: nausea, vomiting, increase in activity of hepatic transaminases, a hyperbilirubinemia, anemia, a leukopenia, a granulocytopenia, thrombocytopenia, an oliguria, a proteinuria, a microhematuria, a renal failure, a headache (migraine), drowsiness, disturbance of neuromuscular conductivity, vestibular disorders, irreversible deafness, skin rash, an itching, a small tortoiseshell, fever, sometimes – a Quincke's edema.
Treatment: it is necessary to cancel drug, for removal of gentamycin from the circulating blood the hemodialysis, especially in cases when function of kidneys is broken is effective. Extent of removal of gentamycin from an organism at peritoneal dialysis is significantly lower, than at a hemodialysis.
The form of release and packing
On 2 ml spill in ampoules of spray filling of neutral glass with a point or a ring of a break.
On each ampoule paste the label from paper label or papers writing.
On 5 or 10 ampoules pack into blister strip packaging from a film of polyvinylchloride and aluminum foil.
Blister strip packagings together with the approved iinstruktion on medical use in the state and Russian languages place in boxes of cardboard for a retail container or corrugated.
To Store storage conditions in the place protected from light, at a temperature not over 300C.
To store out of children's reach!
2 years
not to use a period of storage after an expiration date.
Prescription status
According to the prescription
JSC Khimfarm Producer, the Republic of Kazakhstan
the Owner of the registration certificate
of JSC Khimfarm, the Republic of Kazakhstan
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods) of JSC Khimfarm, Republic of Kazakhstan, Shymkent, Rashidov St., 81 Phone number 7252 (561342) Fax number 7252 (561342)
to Develop the E-mail address of infomed@santo.kz
of GENTAMYCINI SULFAS medicine
the Trade name
Gentamycini sulfas
the International unlicensed
name Gentamycin Dosage Form Solution for injections of 4%, 2 ml
Structure
of 2 ml of solution contain
active agent - Gentamycini sulfas (in terms of
gentamycin) - 80.0 mg,
excipients: sodium metabisulphite, dinatrium edetat, water for injections.
The description
the Transparent, colourless or slightly painted liquid
Pharmacotherapeutic group
Antibacterial drugs for system use. Aminoglikozidny antibacterial drugs. Other aminoglycosides. Gentamycin.
The ATX J01GB03 code
the Pharmacological
Pharmacokinetics Drug properties is administered intramusculary or intravenously. It is quickly and completely soaked up at intramuscular introduction. After intramuscular introduction of Gentamycini sulfas the peak plasma concentration are reached within 30 - 60 minutes, concentration can be defined in plasma from 6 to 12 hours. Linking of gentamycin with proteins of plasma low from 0 to 10%.
Gets into plasma, a lymph, fabrics (a liver, kidneys, lungs), a phlegm, pleural, synovial and peritoneal liquids and through a placental barrier. Concentration in cortical substance of kidneys can sometimes exceed by eight times usual levels in plasma. In bile low concentration are defined that confirms insignificant removal of gentamycin with bile. Badly gets through a blood-brain barrier. Concentration of gentamycin in cerebrospinal fluid low also depend on a dose, existence and expressiveness of inflammation of a meninx.
Gentamycin can collect in plasma and tissues of patients at introduction of high doses for a long time, especially in a renal failure or in malformations of kidneys. As gentamycin is distributed in extracellular liquid, peak plasma concentration at patients with large volumes of extracellular liquid can be lower, than usually.
Removal of gentamycin depends on post-natal age and clearance of creatinine. With increase in post-natal age and increase in a maturity of kidneys gentamycin is removed more quickly. Metabolic changes of gentamycin are minimum, removal happens by glomerular filtration. In several days of therapy the amount of the removed gentamycin is brought closer, but is not equal to quantity of the entered dose. A small part of the entered dose of gentamycin can remain in fabrics, in particular in kidneys. At some patients aminoglycosides were defined in insignificant quantity in urine in several weeks after the end of therapy. The renal clearance of gentamycin is similar to clearance of endogenous creatinine.
It is not metabolized. About 90% of gentamycin are removed in not changed look by kidneys by means of glomerular filtration.
At adults the elimination half-life at normal function of kidneys makes 2-3 hours, children aged from 1 week up to 6 months have 3 - 3.5 h, newborn and premature children with body weight have more than 2 kg – 5.5 h, with body weight less than 1.5 kg – 11.5 h, up to 2 kg - 8 h.
At the increased temperature and anemia the elimination half-life can be shortened. Dose adjustment usually is not required. At patients with heavy burns the elimination half-life can decrease considerably, causing decrease in plasma concentration in comparison with expected when dosing in body weight mg/kg.
With urine it is removed - 70–95% of gentamycin and a small amount - with bile.
At patients with a heavy renal failure the decrease in concentration of gentamycin in urine and its penetration into the struck parenchyma of kidneys is observed. It leads to decrease in removal of drug and increase in risk of nephrotoxic effect of aminoglycosides that it is necessary to take into account at treatment of patients with infections of urinary tract.
A pharmacodynamics
Gentamycini sulfas – an antibiotic of a broad spectrum of activity from group of aminoglycosides, antibacterial agent for topical and parenteral administration. Actively getting through a cellular membrane of bacteria, it is irreversible contacts 30S a subunit of bacterial ribosomes and by that activator protein synthesis oppresses. Gentamycin has bactericidal effect concerning many gram-positive and gram-negative microorganisms, including the so-called steady microbes resistant to other antibiotics.
It is active concerning a wide number of pathogenic bacteriums, including Escherichia coli, Proteus spp., Pseudomonas aeruginosa, Klebsiella spp., Enterobacter spp., Serratia spp., Citrobacter spp., Staphylococcus spp. (including the strains resistant to penicillin and Methicillinum).
Neisseria meningitides, Treponema pallidum, some strains of Streptococcus spp., anaerobic bacteria are resistant to gentamycin.
Does not affect mushrooms, viruses, the elementary.
Indications
- lower respiratory tract infections, bronchitis, pneumonia, pleurisy,
pleura empyemas
- infections of the central nervous system, including meningitis, as a part of
complex therapy
- the heavy complicated urinary tract infections, pyelonephritis, cystitis,
an urethritis, prostatitis
- a bacterial septicaemia, sepsis
- acute cholecystitis, a cholangitis, peritonitis
- purulent infections of skin and soft tissues, a burn infection
- infections of bones and joints
the Route of administration and doses
enter Gentamycini sulfas intramusculary or intravenously (by drop infusion). The mode of dosing is set individually taking into account the body weight of the patient.
Adult: 3 mg/kg/days, in heavy infections - 5 mg/kg/days (to about 80 mg there are each 8 hours).
The average duration of treatment – 6-7 days, as much as possible – 10 days.
The recommended doses are identical both for intramuscular, and for intravenous administration.
Solution for injections of Gentamycini sulfas cannot be mixed with other drugs, it is necessary to enter separately according to ways of introduction and the mode of dosing.
Maximum single dose: 160 mg (for patients with normal function of kidneys).
The maximum daily dose for all age groups: 5 mg/kg/days.
To children up to 3 years drug is appointed only according to vital indications in heavy infections
the Dosage for children of various age taking into account body weight.
Daily doses make:
to newborns and children of chest age – 2 – 5 mg/kg,
to children aged from 1 year up to 5 years – 1.5 - 3 mg/kg, 6 – 14 years – 3 mg/kg. The maximum daily dose for children of all age groups makes 5 mg/kg. The drug is administered by 2 – 3 times a day.
In a renal failure the correction of the mode of dosing is required. When carrying out a hemodialysis the recommended doses after its session for adults - 1-1.7 mg/kg (depending on weight of an infection), for children – 2 - 2.5 mg/kg.
At a heavy course of infections the purpose of smaller single doses with bigger frequency rate is recommended, decrease in size of a single dose has to be multiple to the relation calculated by the interval formula given above to the size of a normal interval between introductions (8 h). The dose has to be picked up so that Cmax did not exceed 12 mkg/ml (reduction of risk of development nefro-, from - and neurotoxicity).
In hypostases, ascites, obesity the dose is determined by 'ideal' or 'dry' body weight.
Whenever possible it is necessary to carry out control of gentamycin level in plasma.
Intravenous administration of the Dose same, as for intramuscular introduction. The usual volume of solvent (sterile normal saline solution or 5% glucose solution) is 100-200 ml for adults, for children the volume of solvent has to be reduced in proportion. Concentration of Gentamycin in solution should not exceed 1 mg/ml (0.1%). Solution is entered in the form of slow infusion within 1-2 hours. In the syringe Gentamycini sulfas cannot be mixed with any other medicine.
Side effects
- a headache, drowsiness, disturbance of neuromuscular conductivity,
muscular pains, spasms, vestibular disorders, decrease in hearing (in certain cases – irreversible deafness)
- nausea, vomiting, loss of appetite, decrease in body weight, the increased salivation
- increase in activity of hepatic transaminases, a hyperbilirubinemia
- anemia, a leukopenia, a granulocytopenia, thrombocytopenia
- an oliguria, a proteinuria, a microhematuria,
- a renal failure
- skin rash, an itching, a small tortoiseshell, fever, sometimes – a Quincke's edema
- a hypocalcemia, a gipokalemiya, a gipomagnemiya (at children)
In rare instances
- tubular necrosis
- an acute anaphylaxis
- morbidity in the place of intramuscular introduction
- phlebitis and thrombophlebitises at intravenous administration
- development of superinfection
of the Contraindication
- hypersensitivity to components of drug and other
antibiotics of group of aminoglycosides
- neuritis of an acoustical nerve
- a disease of a vestibular mechanism
- heavy renal failures, uraemia, an azotemia
- pregnancy and the period of a lactation
- a myasthenia, parkinsonism
- children's age up to 3 years
Medicinal interactions
It is necessary to take into account a possibility of development of neuromuscular blockade and paralysis of breath at any way of administration of aminoglycosides at the patients receiving the anesthetics or means causing neuromuscular blockade such as suktsinilkholin, tubocurarine, dekametony and also at patients to whom massive transfusions of citrated blood are carried out. At approach of neuromuscular blockade enter calcium salts.
Simultaneous or subsequent system or topical administration of other potentially neurotoxic or nephrotoxic drugs, such as Cisplatinum, Cefaloridinum, Kanamycinum, amikacin, Neomycinum, polimiksin-V, colistin, paromomitsin, streptomycin, Tobramycinum, Vancomycinum and Viomycinum is not recommended.
At simultaneous use of a hydrocortisone and indometacin the nephrotoxic effect of gentamycin can amplify.
It is not necessary to apply along with furosemide and Acidum etacrynicum because strengthening of ototoksichesky and nephrotoxic action is possible. Besides, at intravenous use of diuretics the change of concentration of an antibiotic in plasma and fabrics is possible that leads to strengthening of the toxic reactions caused by aminoglycosides.
At the patients with a heavy renal failure receiving at the same time karbenitsillin and gentamycin, decrease in elimination half-life of gentamycin from plasma was observed.
Pharmaceutical it is incompatible with a beta laktamnymi antibiotics, heparins, Amphotericinum.
Special instructions
At drug use whenever possible carry out monitoring of plasma concentration of gentamycin. Increase in an interval between introductions can be required. In view of the fact that concentration of creatinine of plasma have close correlation with elimination half-life of gentamycin, laboratory tests on creatinine can serve as the management for establishment of an interval between introductions.
At use of drug it is necessary to control functions of kidneys, acoustical and vestibular devices.
Gentamycin should be applied with care at patients with botulism, dehydration, parkinsonism, a hypocalcemia and also at patients of advanced age. The risk of ototoxicity increases in dehydration of an organism and at elderly people in this connection they have to use enough liquid. The patients having diseases of kidneys, a hearing loss, dizzinesses or sonitus are especially sensitive to gentamycin.
Introduction of all daily dose of Gentamycini sulfas in burns more than 20%, an endocarditis, sepsis is not recommended.
Drug is not recommended for treatment of not hospital pneumonia, both in out-patient, and in stationary conditions. Against the background of treatment the resistance of microorganisms can develop. In similar cases it is necessary to cancel drug and to appoint treatment on the basis of data of an antibiotikogramma. The bisulphite which is contained in sodium ampoules can cause development in sick allergic complications, especially in patients with the burdened allergic anamnesis.
Use in pediatrics
to Children up to 3 years only according to vital indications in heavy infections.
Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
Considering side effects of drug it is necessary to be careful at control of motor transport or potentially dangerous mechanisms.
Overdose
Symptoms: nausea, vomiting, increase in activity of hepatic transaminases, a hyperbilirubinemia, anemia, a leukopenia, a granulocytopenia, thrombocytopenia, an oliguria, a proteinuria, a microhematuria, a renal failure, a headache (migraine), drowsiness, disturbance of neuromuscular conductivity, vestibular disorders, irreversible deafness, skin rash, an itching, a small tortoiseshell, fever, sometimes – a Quincke's edema.
Treatment: it is necessary to cancel drug, for removal of gentamycin from the circulating blood the hemodialysis, especially in cases when function of kidneys is broken is effective. Extent of removal of gentamycin from an organism at peritoneal dialysis is significantly lower, than at a hemodialysis.
The form of release and packing
On 2 ml spill in ampoules of spray filling of neutral glass with a point or a ring of a break.
On each ampoule paste the label from paper label or papers writing.
On 5 or 10 ampoules pack into blister strip packaging from a film of polyvinylchloride and aluminum foil.
Blister strip packagings together with the approved iinstruktion on medical use in the state and Russian languages place in boxes of cardboard for a retail container or corrugated.
To Store storage conditions in the place protected from light, at a temperature not over 300C.
To store out of children's reach!
2 years
not to use a period of storage after an expiration date.
Prescription status
According to the prescription
JSC Khimfarm Producer, the Republic of Kazakhstan
the Owner of the registration certificate
of JSC Khimfarm, the Republic of Kazakhstan
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods) of JSC Khimfarm, Republic of Kazakhstan, Shymkent, Rashidov St., 81 Phone number 7252 (561342) Fax number 7252 (561342)
to Develop the E-mail address of infomed@santo.kz