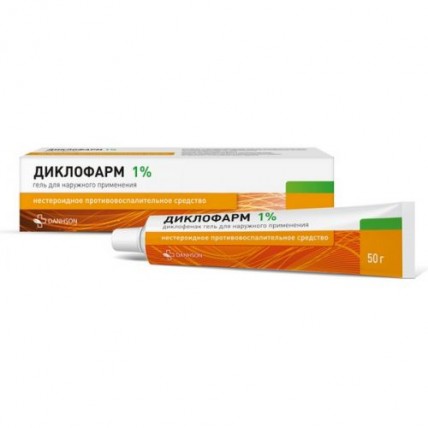
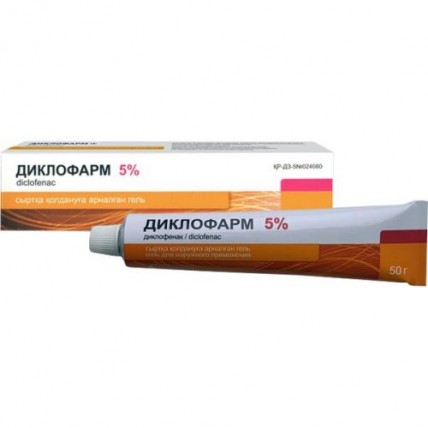
Diclopharm (Diclofenac) Gel 1% / 5%, 50 g
- $6.00
What is Diclopharm Gel and How Does It Work?
Diclopharm Gel, containing Diclofenac as the active ingredient, is a topical anti-inflammatory treatment available in two concentrations: 1% and 5%. It is commonly used to alleviate pain and inflammation in muscles and joints due to injuries, strains, or conditions like osteoarthritis. Available in a 50 g tube, Diclopharm offers fast, targeted relief through local application on the affected area.
What is Diclopharm Gel Made Of?
Each concentration of Diclopharm Gel contains specific amounts of Diclofenac:
- 5% Gel: 100 g contains 5 g of Diclofenac sodium.
- 1% Gel: 100 g contains 1 g of Diclofenac sodium.
Additional ingredients include carbomer, isopropyl alcohol, purified water, and preservatives like methylparahydroxybenzoate to enhance stability.
How Does Diclopharm Gel Work?
Diclofenac, a non-steroidal anti-inflammatory drug (NSAID), works by inhibiting enzymes that contribute to inflammation and pain, specifically cyclooxygenase-1 and -2 (COX-1 and COX-2). This reduces the synthesis of prostaglandins, substances responsible for inflammatory processes. When applied to the skin, Diclofenac is absorbed directly into the painful area, concentrating in joint and muscle tissues to reduce swelling, alleviate discomfort, and improve mobility.
When Should You Use Diclopharm Gel?
Diclopharm Gel is typically recommended for conditions that involve joint and muscle pain, such as:
- Back pain due to inflammatory or degenerative conditions (e.g., sciatica, osteoarthritis).
- Joint pain in areas like fingers and knees, particularly with osteoarthritis.
- Muscle pain from strains, bruises, or overuse.
- Soft tissue inflammation caused by injuries or rheumatic conditions, including tendonitis and bursitis.
This gel is intended for symptomatic relief, helping reduce pain and inflammation during use but does not prevent disease progression.
How to Apply Diclopharm Gel?
For adults and children over 12:
- Apply a thin layer of gel on the inflamed area 2-3 times daily, gently massaging it into the skin.
- Use a maximum single dose of 2 g (approx. 4 cm strip from the tube), with a daily limit of 6 g.
- Avoid contact with broken skin, eyes, or mucous membranes, and wash hands after application unless treating them.
If symptoms persist or worsen after seven days of use, consult a healthcare provider. For safe use, avoid prolonged applications beyond 14 days without medical advice.
Are There Any Side Effects?
Most users experience mild, localized skin reactions like itching, redness, or rash at the application site. Rarely, some may experience allergic reactions, such as urticaria or angioedema, and very rarely, respiratory issues like asthma.
Frequency of side effects:
- Common: Contact dermatitis, including itching, redness, and rashes.
- Rare: Hypersensitivity reactions like hives, swelling, or skin rash.
- Very Rare: Serious allergic responses like anaphylactic shock or asthma.
Who Should Avoid Using Diclopharm Gel?
Do not use Diclopharm if you have:
- Allergy to Diclofenac or similar NSAIDs.
- A history of asthma or allergic reactions to aspirin or other NSAIDs.
- Broken skin in the area where the gel would be applied.
- Pregnancy in the third trimester or are under 12 years old.
Can Diclopharm Gel Interact with Other Medications?
Diclofenac gel may increase sensitivity to sunlight if combined with other medications causing photosensitivity.
No significant drug interactions have been documented, but consult a healthcare provider if using other treatments.
Precautions for Use During Pregnancy and Breastfeeding
- Pregnancy: Only use during the first two trimesters if prescribed by a doctor, weighing the potential risks and benefits. Avoid in the third trimester due to possible risks to the fetus.
- Breastfeeding: Avoid application on the breasts or extensive body areas. Do not use long-term during lactation without consulting a healthcare provider.
How Should Diclopharm Gel Be Stored?
Store Diclopharm Gel in a cool place, at temperatures no higher than 25°C (77°F).
Ensure it is kept out of reach of children.
Once opened, use within 30 days and discard any remaining product after the expiration date.