-25%
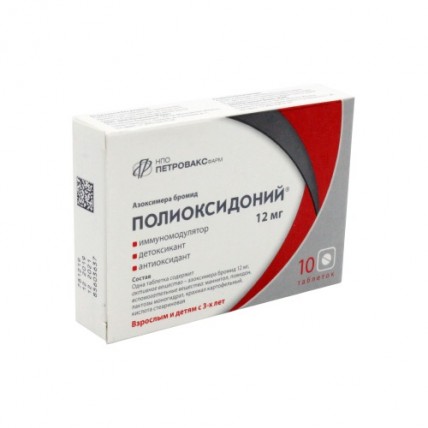
POLYOXIDONIUM® (Azoximer Bromide, AZB) 12 mg, 10 tablets
- $40.00
- $53.00
Compound
One tablet contains
- Active ingredients - Azoximer bromide 12 mg,
- Excipients: mannitol, povidone, lactose monohydrate, potato starch, stearic acid.
Pharmacological properties
Pharmacokinetics
Azoximer bromide after oral administration is rapidly absorbed from the gastrointestinal tract, the bioavailability of the drug when administered orally is more than 70%. The maximum plasma concentration is reached 3 hours after ingestion. The pharmacokinetics of Azoximer bromide is linear (plasma concentration is proportional to the dose taken).
Azoximer bromide is a hydrophilic compound. The apparent volume of distribution is approximately 0.5 l / kg, which indicates that the drug is distributed mainly in the interstitial fluid. The half-life is 35 minutes, the half-life is 18 hours.
Azoximer bromide is rapidly distributed throughout all organs and tissues of the body, penetrates through the blood-brain and hemato-ophthalmic barriers. There is no cumulative effect. In the body of Azoximer, bromide undergoes biodegradation to low molecular weight oligomers, it is excreted mainly by the kidneys, with faeces - no more than 3%.
Pharmacodynamics
Azoximer bromide has a complex effect: immunomodulatory, detoxifying, antioxidant, moderate anti-inflammatory.
The basis of the mechanism of the immunomodulatory action of Azoximer bromide is a direct effect on phagocytic cells and natural killers, as well as stimulation of antibody production and synthesis of interferon-alpha and interferon-gamma.
The detoxifying and antioxidant properties of Azoximer bromide are largely determined by the structure and high-molecular nature of the drug.
Azoximer bromide increases the body's resistance to local and generalized infections of bacterial, fungal and viral etiology. Restores immunity in secondary immunodeficiency states caused by various infections, injuries, complications after surgical operations.
A characteristic feature of Azoximer bromide when applied topically (sublingually) is the ability to activate the factors of early protection of the body against infection: the drug stimulates the bactericidal properties of neutrophils, macrophages, enhances their ability to absorb bacteria, increases the bactericidal properties of saliva and mucous secretion of the upper respiratory tract.
When administered orally, Azoximer bromide also activates lymphoid cells in the intestinal lymph nodes.
Azoximer bromide blocks soluble toxic substances and microparticles, has the ability to remove toxins, salts of heavy metals from the body, inhibits lipid peroxidation, both by intercepting free radicals and by eliminating catalytically active Fe2+ ions. Azoximer bromide reduces the inflammatory response by normalizing the synthesis of pro- and anti-inflammatory cytokines.
Azoximer bromide is well tolerated, has no mitogenic, polyclonal activity, antigenic properties, does not have an allergenic, mutagenic, embryotoxic, teratogenic and carcinogenic effect.
Azoximer bromide is odorless and tasteless, does not have a local irritating effect when applied to the mucous membranes of the nose and oropharynx.
Indications for use
It is used in adults and children from 3 years old for the treatment and prevention of acute and chronic respiratory diseases in the stage of exacerbation and remission.
For treatment (in complex therapy):
- acute and exacerbation of chronic recurrent infectious and inflammatory diseases of the oropharynx, paranasal sinuses, upper and lower respiratory tract, inner and middle ear;
- allergic diseases (including hay fever, bronchial asthma) complicated by recurrent bacterial, fungal and viral infections;
For prevention (monotherapy):
- recurrent herpetic infection of the nasal and labial region;
- exacerbations of chronic foci of infections of the oropharynx, paranasal sinuses, upper respiratory tract, inner and middle ear;
- secondary immunodeficiency states arising from aging or exposure to adverse factors.
Dosage and administration
Use the drug only according to the indications, the method of application and in the doses indicated in the instructions.
If there is no improvement after treatment, or the symptoms worsen, or new symptoms appear, a doctor should be consulted.
Orally and sublingually 20-30 minutes before meals daily 2 times a day: children over 10 years old and adults - 1 tablet, children from 3 to 10 years old - ½ tablet (6 mg).
If necessary, it is possible to conduct repeated courses of therapy after 3-4 months. With repeated administration of the drug, its effectiveness does not decrease.
Recommended treatment regimens
sublingual
For adult treatment:
Influenza and acute respiratory infections - 1 tablet 2 times a day for 7 days;
Inflammatory processes of the oropharynx - 1 tablet 2 times a day for 10 days;
exacerbations of chronic diseases of the upper respiratory tract, paranasal sinuses, chronic otitis - 1 tablet 2 times a day for 10 days;
Allergic diseases (including hay fever, bronchial asthma) complicated by recurrent bacterial, fungal and viral infections - 1 tablet 2 times a day for 10 days.
For the treatment of children from 3 to 10 years:
Influenza and acute respiratory infections - ½ tablet 2 times a day for 7 days;
Inflammatory processes of the oropharynx - ½ tablet 2 times a day for 7 days;
Allergic diseases (including hay fever, bronchial asthma) complicated by recurrent bacterial, fungal and viral infections - ½ tablet 2 times a day for 7 days.
For treatment of children over 10 years of age:
Influenza and acute respiratory infections - 1 tablet 2 times a day for 7 days;
Inflammatory processes of the oropharynx - 1 tablet 2 times a day for 7 days;
exacerbations of chronic diseases of the upper respiratory tract, paranasal sinuses, chronic otitis - 1 tablet 2 times a day for 7 days;
Allergic diseases (including hay fever, bronchial asthma) complicated by recurrent bacterial, fungal and viral infections - 1 tablet 2 times a day for 7 days.
For prevention in adults:
Influenza and acute respiratory infections in the pre-epidemic period - 1 tablet per day for 10 days;
Recurrent herpetic infection of the nasal and labial area - 1 tablet 2 times a day for 10 days;
Exacerbations of chronic foci of infections of the oropharynx, paranasal sinuses, upper respiratory tract, inner and middle ear - 1 tablet once a day for 10 days;
Secondary immunodeficiencies arising from aging or exposure to adverse factors - 1 tablet 1 time per day for 10 days.
For prevention for children from 3 to 10 years:
Influenza and acute respiratory infections in the pre-epidemic period - ½ tablet per day for 7 days;
Recurrent herpetic infection of the nasal and labial area - ½ tablet 2 times a day for 7 days;
Exacerbations of chronic foci of infections of the oropharynx, paranasal sinuses, upper respiratory tract, inner and middle ear - ½ tablet once a day for 10 days.
For prevention in children over 10 years of age:
Influenza and acute respiratory infections in the pre-epidemic period - 1 tablet per day for 7 days;
Recurrent herpetic infection of the nasal and labial area - 1 tablet 2 times a day for 7 days;
Exacerbations of chronic foci of infections of the oropharynx, paranasal sinuses, upper respiratory tract, inner and middle ear, 1 tablet once a day for 10 days.
oral
For adult treatment:
diseases of the upper and lower respiratory tract - 1 tablet 2 times 10 days.
For treatment of children over 10 years of age:
diseases of the upper and lower respiratory tract - 1 tablet 2 times 10 days.
Possible side effects
Side effects are not registered.
If you notice any side effects that are not listed in the instructions, tell your doctor.
Contraindications
- Increased individual sensitivity;
- Pregnancy, breastfeeding period;
- Children's age up to 3 years;
- acute renal failure;
- rare hereditary lactose intolerance, lactase deficiency, glucose-galactose malabsorption syndrome.
Carefully
If you have any of the conditions listed in this section, talk to your doctor before you start taking this medicine:
- chronic renal failure (used no more than 2 times a week).
Drug Interactions
Azoximer bromide does not inhibit isoenzymes CYP1A2, CYP2C9, CYP2C19, CYP2D6, cytochrome P-450, so the drug is compatible with antibiotics, antiviral, antifungal and antihistamines, glucocorticosteroids and cytostatics.
If you are taking any of the above or other medicines (including over-the-counter medicines), talk to your doctor before taking Polyoxidonium.
Special instructions
Please read this leaflet carefully before you start using this medicine as it contains important information for you.
Save the instructions, they may be needed again.
If you have any questions, please contact your doctor.
This medicine is available without a prescription. To achieve optimal results, it should be used strictly following all the recommendations outlined in the instructions.
The medicine you are taking is for you personally and should not be passed on to others as it may harm them even if you have the same symptoms as you.
If an allergic reaction develops, stop using Polyoxidonium® and consult a doctor.
If it is necessary to stop taking the drug Polyoxidonium, cancellation can be carried out immediately, without a gradual dose reduction.
If you miss the next dose of the drug, its subsequent use should be carried out as usual, as indicated in this leaflet or recommended by your doctor. The patient should not double the dose to make up for missed doses.
Do not use the drug if there are visual signs of its unsuitability (packaging defect, discoloration of the tablet).
Use during pregnancy and lactation
The use of the drug Polyoxidonium® is contraindicated in pregnant women and women during breastfeeding (clinical experience is not available).
Before using the drug Polyoxidonium®, if you are pregnant, or think that you could be pregnant, or are planning to become pregnant, you should consult your doctor.
During breastfeeding, before using the drug Polyoxidonium, you should consult your doctor.
Features of the influence of the drug on the ability to drive vehicles or potentially dangerous mechanisms
The use of the drug Polyoxidonium® does not affect the ability to perform potentially hazardous activities that require increased concentration of attention and speed of psychomotor reactions (including driving, working with moving mechanisms).
Storage conditions
Store at a temperature not exceeding 25 °C.
Keep out of the reach of children!
Shelf life - 2 years.
Do not use after the expiration date.