Instruction for medical use
of PLE-SPA™ medicine
Trade name
of Ple-spa™
International unlicensed
name Drotaverinum Dosage Form
of the Tablet, film coated
Ingredients:
One tablet contains
active agent - Drotaverinum a hydrochloride of 40 mg,
excipients: lactose monohydrate, calcium phosphate double-base, sodium of starch stearate,
structure of a cover glikolit (primogel), sodium lauryl sulfate, silicon dioxide colloidal (aerosil-200), povidone, calcium hydrophosphate anhydrous, magnesium: instakoat water – III white (IA – III 40001), quinolinic yellow (E 104).
The description
of the Tablet, film coated, yellow color, round with a biconvex surface.
Pharmacotherapeutic group
Drugs for treatment of functional disorders of a GIT. Papaverine and its derivatives.
The code of automatic telephone exchange A03AD02
the Pharmacological
Pharmacokinetics Later properties of introduction of a single oral dose of 80 mg, peak plasma concentration of blood are reached in 1-3 hours (on average, in 1.9 hours), and their values fluctuate in the range from 136 - 409 ng/ml, and average area under a curve concentration – time is (AUC) 3251 ng/ml/hour. Time of semi-removal of Drotaverinum fluctuates from 7 to 12 hours. About 90% of the appointed dose are soaked up from a small intestine within 60 minutes. Drotaverinum and its metabolites contact proteins of blood plasma for 80-95%. Spasmolytic action is mediated by not changed substance, begins in 30 minutes in most cases and continues within 4 hours. Drotaverinum is extensively metabolized in a liver and removed with urine and excrements. The renal clearance is 0.21 - 1.28 ml a minute.
A pharmacodynamics
of Ple-spa™ – the synthetic spasmolysant of myotropic action eliminating spasms of smooth muscles of internals and vessels.
Spasmolytic effect of drug is mediated by suppression of effect of the enzyme of phosphodiesterase-IV specific to smooth muscles. Drug has fast direct effect on unstriated muscles that is connected with increase in concentration ts-AMF and elimination of a calcic imbalance in the spastic site. Drug is effective at spasms of smooth muscles of digestive tract, urinary tract, a uterus, vessels.
Indications
- spasms of smooth muscles of digestive tract: a peptic ulcer of a stomach and duodenum, spasms of peloric and cardial department of a stomach, bilious colic and spasms in cholelithiasis, cholecystitis, a cholangitis, a spastic constipation, spasms in colitis and a meteorism, tenesmus
- renal colic in a nephrolithiasis, pyelonephritis, spastic pains in cystitis, a pyelitis and prostatitis
- spasms of smooth muscles when holding diagnostic procedures
- a postoperative meteorism of spastic origin
- algodismenoreya, for weakening of reductions of a uterus at the menacing abortion and premature births, spasmolysis of a neck of the uterus at childbirth.
Route of administration and doses:
Adult: on 40-80 mg three times a day.
Maximum single dose: 80 mg.
Maximum daily dose: 240 mg.
Duration of treatment is defined by the doctor, considering character and severity of a disease.
Side effects
- hypotension, tachycardia
- a headache, dizziness
- allergic reactions
Seldom
- ruptures of a neck of the uterus
-
Contraindication nausea
- hypersensitivity to Drotaverinum and other components of drug
- glaucoma
- the profound atherosclerosis of coronary arteries
- prostate adenoma
- heavy renal, liver and heart failure
- AV blockade 2-3 degrees
- the children's age up to 18 years
Medicinal interactions
Strengthens the lowering of arterial pressure caused by tricyclic antidepressants, quinidine, procaineamide. It is necessary to show care at simultaneous use with a levodopa in parkinsonism as the antiparkinsonichesky effect of a levodopa decreases. Simultaneous use of a papaverine, Bendazolum, anesthetics, antimuskarinovy means or benzodiazepines leads to strengthening of their action. Reduces spazmogenny activity of morphine.
The special
instructions Pregnancy and the period of a lactation
during pregnancy drug is used according to indications.
There are no messages about use of Ple-spa™ in the period of a lactation. If necessary treatment has to be carried out only under medical observation, it is necessary to resolve an issue of the termination or continuation of breastfeeding.
Patients with a renal failure or a liver
dose adjustment at patients with a renal failure or a liver as Drotaverinum and its metabolites are exposed to intensive metabolism in a liver Is necessary and are removed through kidneys.
Features of influence of drug on ability to run the vehicle or potentially dangerous mechanisms
Considering side effects of drug (dizziness, a headache) it is necessary to be careful at control of transport or other potentially dangerous mechanisms.
Overdose
Symptoms: headache, dizziness, lowering of arterial pressure, tachycardia, nausea.
Treatment: at overdose appoint gastric lavage, activated carbon inside. Symptomatic treatment is shown.
A form of release and packing
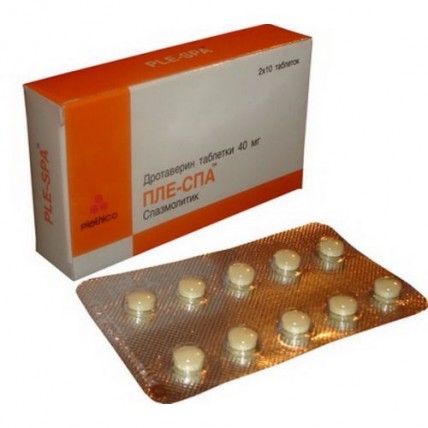
of 10 tablets in blister strip packaging.
On 1 or 2 blister strip packagings in cardboard packing with the instruction for medical use in the state and Russian languages.
To Store storage conditions in the dry, protected from light place at a temperature not above 25 °C.
To store out of children's reach!
3 years
not to apply a period of storage after an expiration date.
Prescription status
According to the prescription
Plethico Pharmaceuticals Ltd/Pletkhiko Pharmasyyutikalz Ltd Adres Producer of location:
Dharavara, Kalariya – 453001, Indore (M.item.), India.
Legal address:
37/37A, Indastrial Isteyt, Polograund, Indore (L. S.), 452,015, India
the Address of the organization accepting in the territory of the Republic of Kazakhstan consumers on quality of products
Representation in RK Rezlov LTD LLP, Karaganda, Ermekov St. 116 A, office 10.
Ph. +7 (212) 442220, fax + 7 (212) 442221.
E-mail address of rezlov_ltd@mail.ru
of PLE-SPA™ medicine
Trade name
of Ple-spa™
International unlicensed
name Drotaverinum Dosage Form
of the Tablet, film coated
Ingredients:
One tablet contains
active agent - Drotaverinum a hydrochloride of 40 mg,
excipients: lactose monohydrate, calcium phosphate double-base, sodium of starch stearate,
structure of a cover glikolit (primogel), sodium lauryl sulfate, silicon dioxide colloidal (aerosil-200), povidone, calcium hydrophosphate anhydrous, magnesium: instakoat water – III white (IA – III 40001), quinolinic yellow (E 104).
The description
of the Tablet, film coated, yellow color, round with a biconvex surface.
Pharmacotherapeutic group
Drugs for treatment of functional disorders of a GIT. Papaverine and its derivatives.
The code of automatic telephone exchange A03AD02
the Pharmacological
Pharmacokinetics Later properties of introduction of a single oral dose of 80 mg, peak plasma concentration of blood are reached in 1-3 hours (on average, in 1.9 hours), and their values fluctuate in the range from 136 - 409 ng/ml, and average area under a curve concentration – time is (AUC) 3251 ng/ml/hour. Time of semi-removal of Drotaverinum fluctuates from 7 to 12 hours. About 90% of the appointed dose are soaked up from a small intestine within 60 minutes. Drotaverinum and its metabolites contact proteins of blood plasma for 80-95%. Spasmolytic action is mediated by not changed substance, begins in 30 minutes in most cases and continues within 4 hours. Drotaverinum is extensively metabolized in a liver and removed with urine and excrements. The renal clearance is 0.21 - 1.28 ml a minute.
A pharmacodynamics
of Ple-spa™ – the synthetic spasmolysant of myotropic action eliminating spasms of smooth muscles of internals and vessels.
Spasmolytic effect of drug is mediated by suppression of effect of the enzyme of phosphodiesterase-IV specific to smooth muscles. Drug has fast direct effect on unstriated muscles that is connected with increase in concentration ts-AMF and elimination of a calcic imbalance in the spastic site. Drug is effective at spasms of smooth muscles of digestive tract, urinary tract, a uterus, vessels.
Indications
- spasms of smooth muscles of digestive tract: a peptic ulcer of a stomach and duodenum, spasms of peloric and cardial department of a stomach, bilious colic and spasms in cholelithiasis, cholecystitis, a cholangitis, a spastic constipation, spasms in colitis and a meteorism, tenesmus
- renal colic in a nephrolithiasis, pyelonephritis, spastic pains in cystitis, a pyelitis and prostatitis
- spasms of smooth muscles when holding diagnostic procedures
- a postoperative meteorism of spastic origin
- algodismenoreya, for weakening of reductions of a uterus at the menacing abortion and premature births, spasmolysis of a neck of the uterus at childbirth.
Route of administration and doses:
Adult: on 40-80 mg three times a day.
Maximum single dose: 80 mg.
Maximum daily dose: 240 mg.
Duration of treatment is defined by the doctor, considering character and severity of a disease.
Side effects
- hypotension, tachycardia
- a headache, dizziness
- allergic reactions
Seldom
- ruptures of a neck of the uterus
-
Contraindication nausea
- hypersensitivity to Drotaverinum and other components of drug
- glaucoma
- the profound atherosclerosis of coronary arteries
- prostate adenoma
- heavy renal, liver and heart failure
- AV blockade 2-3 degrees
- the children's age up to 18 years
Medicinal interactions
Strengthens the lowering of arterial pressure caused by tricyclic antidepressants, quinidine, procaineamide. It is necessary to show care at simultaneous use with a levodopa in parkinsonism as the antiparkinsonichesky effect of a levodopa decreases. Simultaneous use of a papaverine, Bendazolum, anesthetics, antimuskarinovy means or benzodiazepines leads to strengthening of their action. Reduces spazmogenny activity of morphine.
The special
instructions Pregnancy and the period of a lactation
during pregnancy drug is used according to indications.
There are no messages about use of Ple-spa™ in the period of a lactation. If necessary treatment has to be carried out only under medical observation, it is necessary to resolve an issue of the termination or continuation of breastfeeding.
Patients with a renal failure or a liver
dose adjustment at patients with a renal failure or a liver as Drotaverinum and its metabolites are exposed to intensive metabolism in a liver Is necessary and are removed through kidneys.
Features of influence of drug on ability to run the vehicle or potentially dangerous mechanisms
Considering side effects of drug (dizziness, a headache) it is necessary to be careful at control of transport or other potentially dangerous mechanisms.
Overdose
Symptoms: headache, dizziness, lowering of arterial pressure, tachycardia, nausea.
Treatment: at overdose appoint gastric lavage, activated carbon inside. Symptomatic treatment is shown.
A form of release and packing
of 10 tablets in blister strip packaging.
On 1 or 2 blister strip packagings in cardboard packing with the instruction for medical use in the state and Russian languages.
To Store storage conditions in the dry, protected from light place at a temperature not above 25 °C.
To store out of children's reach!
3 years
not to apply a period of storage after an expiration date.
Prescription status
According to the prescription
Plethico Pharmaceuticals Ltd/Pletkhiko Pharmasyyutikalz Ltd Adres Producer of location:
Dharavara, Kalariya – 453001, Indore (M.item.), India.
Legal address:
37/37A, Indastrial Isteyt, Polograund, Indore (L. S.), 452,015, India
the Address of the organization accepting in the territory of the Republic of Kazakhstan consumers on quality of products
Representation in RK Rezlov LTD LLP, Karaganda, Ermekov St. 116 A, office 10.
Ph. +7 (212) 442220, fax + 7 (212) 442221.
E-mail address of rezlov_ltd@mail.ru