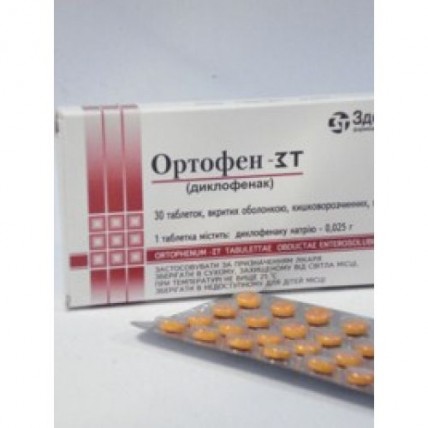
Ortophenum-GP 30s 25 mg coated tablets
- $3.20
Out Of Stock
The instruction
for medical use
of ORTOPHENUM-HEALTH medicine
the Trade name
of Ortofen-Zdorovye
the International unlicensed
name Diclofenac Dosage Form
of the Tablet, covered with a kishechnorastvorimy cover, 25 mg
Structure
One tablet contains
active agent of diclofenac sodium (in terms of 100% substance) 25 mg,
excipients: lactoses monohydrate, cellulose microcrystalline, krospovidon, sodium of a kroskarmelloz, magnesium stearate, silicon dioxide colloidal anhydrous,
cover: dry mix Acryl-eze white (talc, titan dioxide (E 171), methacrylate copolymer (type C), sodium lauryl sulfate, Natrii hydrocarbonas, silicon dioxide colloidal anhydrous), macrogoal 6000, dye bright red 4R Ponso 4R E 124, dye yellow decline of FCF E 110.
The description
of the Tablet, coated, from salmoncolored till pink-orange color. On a surface of tablets the insignificant marbling is allowed. On cross section two layers are visible.
Pharmacotherapeutic group
Anti-inflammatory and antirheumatic drugs. Non-steroidal anti-inflammatory drugs. Acetic acid derivatives. Diclofenac
ATX M01AB05 Code
Pharmacological
Pharmacokinetics Absorption properties. At intake, diclofenac is quickly and completely absorbed. The concomitant use of food reduces absorption speed, without changing amount of the absorbed active ingredient.
The maximum concentration in blood (Cmax) after single use in diclofenac in a dose of 50 mg is reached approximately in 2 hours and makes 1.5 mkg/ml (5 µmol/l). The amount of the absorbed active ingredient is proportional to a dose.
About a half of a dose of diclofenac is metabolized during the first passing through a liver (effect of the first passing). In case of observance of the recommended interval of dosing of accumulation of drug it is not observed.
Distribution. Communication with proteins of plasma more than 99%. The expressed volume of distribution is from 0.12 to 0.17 l/kg. Diclofenac gets into synovial fluid where reaches the maximum concentration in 3-6 hours. In 2 hours after achievement of peak concentration in blood plasma the levels of active agent in synovial fluid are already higher, than in plasma, and remain higher till 12 o'clock.
Biotransformation. It is metabolized in a liver partially by a glyukuronidation of an initial molecule, but mainly due to single and multiple hydroxylation and a metoksilirovaniye that leads to formation of several phenolic metabolites, the majority of which are converted into glyukuronidny conjugates. Two of these phenolic metabolites are biologically active, but to a lesser extent, than diclofenac.
Elimination. The general system clearance of diclofenac from plasma of 263 ml/min. Elimination half-life (T½) from plasma about 2 hours, from synovial fluid of 3-6 hours. It is removed by kidneys (40-65%), with bile and a stake (35%).
Pharmacokinetics at various groups of patients. Significant differences in absorption, metabolism or removal of drug depending on age of patients are not observed.
At patients with a renal failure the accumulation of not changed active ingredient is not allowed.
At patients with chronic hepatitis or nedekompensirovanny cirrhosis pharmacokinetic parameters of diclofenac same, as well as at the patients who do not have liver diseases.
Pharmacodynamics
Anti-inflammatory, analgetic, febrifuge.
The mechanism of effect of diclofenac is caused by inhibition of cyclooxygenases (TsOG-1 and TsOG-2) therefore reactions of an arachidonic cascade are blocked and synthesis of prostaglandins (PGE2 and PGF2alfa), A2 thromboxane, prostacyclin, leukotrienes and emission of lizosomalny enzymes and also suppression of aggregation of thrombocytes is broken. Diclofenac eliminates or considerably reduces expressiveness of symptoms of inflammation, reduces the hypersensitivity of nerve terminations induced by prostaglandins to biologically active agents which are formed in the inflammation center, lowers the body temperature (preventing effect of prostaglandins on a hypothalamic link of thermal control), reduces concentration of prostaglandins in menstrual blood and intensity of pain at primary dysmenorrhea.
In rheumatic diseases the anti-inflammatory and analgetic properties of diclofenac provide considerable reduction of expressiveness of such symptoms and complaints as a rest pain and at the movement, the morning constraint, a swelling of joints and also improves functions of joints.
At the posttraumatic and postoperative inflammatory phenomena diclofenac quickly stops both spontaneous pain, and an oxycinesia, reduces inflammatory hypostasis and hypostasis of a postoperative wound.
In researches on cellular cultures sodium diclofenac in concentration, equivalent subjects which are reached at treatment of patients does not suppress biosynthesis of proteoglycans of cartilaginous tissue.
Diclofenac shows the significant analgeziruyushchy effect in moderate and severe pain of not rheumatic origin (including at migraine attacks), is capable to eliminate pain and to reduce expressiveness of blood losses at primary dysmenorrhea.
Indications
- inflammatory and degenerative forms of rheumatic diseases (the pseudorheumatism ankylosing a spondylitis, an osteoarthrosis, spondylarthrites), pain syndromes with localization in a backbone, not joint rheumatism
- bad attacks of gout
- the posttraumatic and postoperative pain syndromes which are followed by inflammation and hypostases, for example after dental and orthopedic interventions
- the gynecologic diseases which are followed by a pain syndrome and inflammation, for example primary dysmenorrhea or an adnexitis
- in combination therapy of ENT organs in the serious inflammatory illness which is followed by pain (a pharyngotonsillitis, otitis and others)
Appoint the Route of administration and doses the adult inside in time or at once after a meal, without chewing, washing down with enough water.
The dose is recommended to be selected individually and also to accept the lowest effective dose during shorter time.
For adults the recommended initial daily dose makes 100-150 mg. In mild cases, as well as at long-term therapy, there is usually enough purpose of 75-100 mg.
The general daily dose for adults is usually divided into 2-3 receptions. The daily dose of drug should not exceed 150 mg.
At primary dysmenorrhea the daily dose is selected individually, and usually it makes 50-150 mg. The initial dose can make 50-100 mg, but in case of need it is possible to raise it during several menstrual cycles, but not above than to 200 mg/days. Use of drug should be begun after emergence of the first painful symptoms, duration, depending on symptomatology as soon as possible, makes up to several days.
Single and daily doses and also duration of treatment is established individually by the doctor.
Side effects
Often (from & gt, 1/100 to & lt, 1/10)
- a headache, dizziness
- vertigo
- nausea, vomiting, diarrhea, dyspepsia, abdominal pain, a meteorism, anorexia
- increase in level of transaminases
- rash
Seldom (from & gt, 1/10000 to & lt, 1/1000)
- reactions of hypersensitivity, anaphylactic/anaphylactoid reactions, including hypotension and shock
- drowsiness
- asthma (including dispnoe)
- gastritis, gastrointestinal bleeding (a hematemesis, a melena, diarrhea with blood impurity), ulcer of stomach and intestines, followed or not followed by bleeding or perforation
- hepatitis, jaundice, an abnormal liver function
- urticaria, irritations of skin
Very seldom (& lt, 1/10000)
- thrombocytopenia, a leukopenia, hemolytic anemia, aplastic anemia, an agranulocytosis
- a Quincke's disease
- a disorientation, a depression, insomnia, nightmares, irritability, psychotic disorders
- paresthesia, disturbance of memory, a spasm, alarm, a tremor, aseptic meningitis, a dysgeusia, an acute disorder of cerebral circulation
- a disorder of vision, the obscured sight, a diplopia
- a ring in ears, a hearing disorder
- a cardiopalmus, a stethalgia, heart failure, a myocardial infarction
- hypertensia, a vasculitis
- pneumonia
- colitis (including hemorrhagic colitis and exacerbation of ulcer colitis or Crohn's disease), a constipation, stomatitis, a glossitis, disturbance from a gullet, diafragmopodobny intestinal strictures, pancreatitis
- lightning hepatitis, necrotic hepatitis, a liver failure
- bullous dermatitis, eczema, an erythema, a multiformny erythema, Stephens-Johnson's syndrome, a toxic epidermal necrolysis (Lyell's disease), exfoliative dermatitis, an alopecia, reaction of photosensitivity, purple, purple of Shenleyna-Genokh, an itching
- an acute renal failure, a hamaturia, a proteinuria, a nephrotic syndrome, interstitial nephrite, papillary necrosis
of the Contraindication
- hypersensitivity to diclofenac and any other ingredients of drug
- a peptic ulcer of a stomach or intestines, bleeding or perforation
- hereditary intolerance of fructose, deficiency of Lapp-lactases enzyme, glucose malabsorption - galactoses
- pregnancy and the period of a lactation
- heavy abnormal liver functions, kidneys and heart
- existence in the anamnesis of attacks of asthma, urticaria, acute rhinitis on intake of acetylsalicylic acid or other non-steroidal anti-inflammatory drugs
- diseases of the haematogenic system
- treatment of postoperative pain after operation of coronary shunting or use of the cardiopulmonary bypass
- children's age up to 18 years (because of presence of dyes)
Medicinal interactions
Diclofenac can increase concentration of lithium and digoxin in blood plasma. Monitoring of the last in blood plasma is recommended.
Diclofenac, as well as other non-steroidal anti-inflammatory drugs (NPVP), at combined use with diuretics or antihypertensive drugs (for example - blockers, APF inhibitors) can reduce their antihypertensive effect. Therefore it is necessary to appoint a combination of such drugs with care, and patients (especially advanced age) should control arterial blood pressure periodically. Patients should use enough water, and after the beginning and after the end of the accompanying therapy it is necessary to control periodically function of kidneys, in particular at use of diuretics and APF inhibitors, owing to the increased risk of emergence of nephrotoxicity.
Simultaneous use of kaliysberegayushchy diuretics can lead to increase in level of potassium in blood serum (in case of such combination of medicines this indicator should be controlled often).
Combined use of diclofenac, as well as other system non-steroidal anti-inflammatory drugs, or corticosteroids can increase the frequency of the undesirable phenomena from a digestive tract.
At the patients applying at the same time diclofenac, anticoagulants and antitrombotichesky drugs the increase in risk of bleedings is possible. Therefore in case of such combination of medicines the careful and regular observation of patients is recommended.
Simultaneous use of system non-steroidal anti-inflammatory drugs and selective serotonin reuptake inhibitors can increase risk of gastrointestinal bleeding.
Simultaneous use of diclofenac and antidiabetic drugs is possible, at the same time the efficiency of the last does not change. However in such cases the development of both a hypoglycemia, and a hyperglycemia is very seldom possible that causes need of change of a dose of glucose-lowering drugs during drug use. For this reason it is recommended to control during therapy glucose level in blood.
It is necessary to be careful at diclofenac use less than in 24 hours prior to or after reception of a methotrexate as in such cases the concentration of a methotrexate in blood can increase and amplify its toxic action.
Simultaneous use of diclofenac, a kolestipol or holestiramin reduces diclofenac absorption approximately by 30% and 60% respectively. The drugs should be taken at an interval of several hours.
The drugs stimulating microsomal enzymes of a liver, for example rifampicin, carbamazepine, Phenytoinum, a St. John's wort (Hypericum perforatum) are theoretically capable to reduce concentration of diclofenac in blood plasma.
Influence of diclofenac on synthesis of prostaglandins in kidneys can increase nephrotoxicity of cyclosporine. Therefore it is necessary to appoint drug in smaller doses, than those which would be appointed to the patients who are not accepting cyclosporine.
At the patients applying at the same time antibacterial agents derivative a hinolon and diclofenac the development of spasms is seldom possible.
Special instructions
Impact on digestive tract
At use of all NPVP, including diclofenac, are known cases of development of gastrointestinal bleeding, an ulceration or perforation which can be lethal and arise at any time at patients with existence or lack of the previous symptoms, serious gastrointestinal diseases in the anamnesis. The risk of gastrointestinal bleeding is increased at increase in a dose of NPVP, at patients with an ulcer in the anamnesis, especially, complicated bleeding or perforation and also at elderly patients. For reduction of risk of gastrointestinal toxicity at patients with digestive tract diseases in the anamnesis, stomach cankers, ulcer colitis or Crohn's disease and also at elderly patients, treatment has to be carried out with use of a minimal effective dose of drug and under careful medical observation. The extra care is recommended to be observed at patients who receive at the same time the medicines increasing risk of an ulceration or bleeding such as system corticosteroids, anticoagulants, antithrombocytic means or selective serotonin reuptake inhibitors. For patients with the increased risk of development of gastrointestinal complications and also for patients, receiving therapy by low doses of acetylsalicylic acid, it is necessary to consider the possibility of use of combination therapy of diclofenac, for example, with inhibitors of the protonew pump or mizoprostoly.
In case of development in patients of gastrointestinal bleeding or an ulceration of Ortofen-Zdorovye it is necessary to cancel.
Patients with gastrointestinal disturbances in the anamnesis, especially elderly patients, have to report about any abdominal symptoms (especially about gastrointestinal bleeding).
Patients with respiratory diseases
At patients with asthma, seasonal allergic rhinitis, puffiness of a mucous membrane of a nose, chronic obstructive diseases of lungs or persistent infections of airways (especially if they are connected with symptoms similar to allergic rhinitis) a Quincke's edema or a small tortoiseshell are noted more often in comparison with other patients. To these patients and also patients with an allergy to other drugs (rash, an itching or urticaria), when prescribing the drug Ortofen-Zdorovye it is necessary to observe extra care.
Skin reactions
Very seldom at use of NPVP, including diclofenac, can arise serious skin reactions, including lethal (exfoliative dermatitis, Stephens-Johnson's syndrome and a toxic epidermal necrolysis). Risk of development of such reactions at patients, in most cases, is increased within the first month of treatment. It is necessary to stop use of the drug Ortofen-Zdorovye at appearance of skin rash, injuries of a mucous membrane or other signs of hypersensitivity.
As well as in a case with other NPVP, at drug Ortofen-Zdorovye use the allergic reactions, including anaphylactic/anaphylactoid reactions, can develop in rare instances at patients at whom allergic reactions to diclofenac were not observed earlier.
Impact on a liver
Careful medical observation is necessary when prescribing the drug Ortofen-Zdorovye for patients with an abnormal liver function as their state can worsen.
During long-term treatment the drug Ortofen-Zdorovye, as a precautionary measure, recommends to carry out regular monitoring of function of a liver. As increase in activity of liver enzymes can cause other NPVP, diclofenac. At preservation or further increase in activity of liver enzymes, development of clinical symptoms of disturbance of hepatic function or emergence of other symptoms (for example, eosinophilias, rashes), it is necessary to cancel administration of drug. It must be kept in mind that hepatitis against the background of use of diclofenac can develop without the previous symptoms.
It is necessary to be careful when prescribing the drug Ortofen-Zdorovye to patients with a hepatic porphyria as drug can provoke a porphyria attack.
Impact on kidneys
As cases of a delay of liquid and hypostases at treatment of NPVP, including diclofenac are known, the extra care needs to be observed at patients with dysfunctions of heart or kidneys, with hypertensia in the anamnesis, at elderly patients, patients receiving the accompanying treatment by diuretics or medicines which can make considerable impact on function of kidneys and also at patients with significant reduction of volume of extracellular liquid for any reason to or after big operation. At use of the drug Ortofen-Zdorovye in such cases, as a precautionary measure, it is recommended to carry out monitoring of function of kidneys. Drug phase-out usually leads to restoration of function of kidneys to initial level.
Impact on the cardiovascular
Treatment of NPVP system, including diclofenac, especially in high doses and can be associated for a long time with small increase in risk of development of the serious cardiovascular trombotichesky phenomena (including a myocardial infarction and a stroke). To minimize potential risk of the undesirable cardiovascular phenomena at the patients accepting NPVP, especially in case of existence of factors of cardiovascular risk the smallest effective dose during the shortest possible span has to be used.
Hematologic effects
during long-term treatment diclofenac, as well as other NPVP, recommend to carry out control of all-complete blood count test. As well as other NPVP, diclofenac can temporarily reduce aggregation of thrombocytes. For patients with disturbances of a hemostasis it is necessary to establish careful observation, systematic control of a picture of peripheral blood is necessary.
Geriatrics
elderly patients need to be careful when prescribing drug. In particular, are recommended to appoint the smallest effective dose the weakened elderly patients or patients with low body weight.
Diclofenac, thanks to the pharmakodinamichesky properties, can mask the complaints and symptoms characteristic of infectious and inflammatory diseases.
Pregnancy and the period of a lactation
Use of diclofenac for pregnant women was not studied. Therefore Ortofen-Zdorovye should not appoint throughout the two first trimesters of pregnancy if only the advantage of its use does not exceed risks for a fruit. As well as for other NPVP, use of drug throughout the third trimester of pregnancy is contraindicated owing to risk of development of weakness of patrimonial activity of a uterus and/or premature closing of an arterial channel.
As well as other NPVP, diclofenac in small amounts are allocated in breast milk. Thus, Ortofen-Zdorovye should not be applied during feeding by a breast to prevent undesirable reactions at the child.
As well as other NPVP, diclofenac can negatively affect female fertility therefore the women planning pregnancy are not recommended to appoint drug. At the women having problems with conception or passing a research on infertility it is necessary to consider expediency of drug withdrawal of Ortofen-Zdorovye.
The feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
to the Patients who are feeling dizzy during time reception Ortofena-Zdorovye or other unpleasant feelings from the central nervous system, including disorders of vision, should not drive the car or to operate mechanisms.
Overdose
Symptoms: vomiting, gastrointestinal bleedings, diarrhea, dizziness, a ring in ears or spasms. In case of the profound poisoning the development of an acute renal failure and damages of a liver is possible.
Treatment: gastric lavage, intake of adsorbent. If necessary the supporting and symptomatic therapy which is shown at such complications as arterial hypotension, a renal failure, spasms, disturbances from a digestive tract and respiratory depression. It is improbable that an artificial diuresis, the hemodialysis or hemoperfusion will be useful to diclofenac removal as active agents of non-steroidal anti-inflammatory drugs substantially contact proteins of blood plasma and are exposed to intensive metabolism.
A form of release and packing
On 10 or 30 tablets in blister strip packaging from a film of polyvinylchloride and aluminum foil.
1 blister strip packaging on 30 tablets or 3 blister strip packagings on 10 tablets together with the instruction for medical use in a box of cardboard.
To Store storage conditions in original packing at
a temperature not higher than 25 S. Hranit out of children's reach!
A period of storage
3 years
not to use drug after the expiration date specified on packing!
Prescription status
According to the prescription
LLC Pharmatsevticheskaya Producer the Health company.
Ukraine, 61013, Kharkiv, Shevchenko St., 22.
Owner of the registration certificate
of LLC Pharmatsevticheskaya Health company, Ukraine.
The address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods)
of limited liability partnership of Pharmaceutical Euro
050039, Almaty, Mailing St., 72, apartment 34
Ph.: +7 (727) 271-10-17
Fax: +7 (727) 271-84-97
E-mail:
To develop farmevro@mail.ru
for medical use
of ORTOPHENUM-HEALTH medicine
the Trade name
of Ortofen-Zdorovye
the International unlicensed
name Diclofenac Dosage Form
of the Tablet, covered with a kishechnorastvorimy cover, 25 mg
Structure
One tablet contains
active agent of diclofenac sodium (in terms of 100% substance) 25 mg,
excipients: lactoses monohydrate, cellulose microcrystalline, krospovidon, sodium of a kroskarmelloz, magnesium stearate, silicon dioxide colloidal anhydrous,
cover: dry mix Acryl-eze white (talc, titan dioxide (E 171), methacrylate copolymer (type C), sodium lauryl sulfate, Natrii hydrocarbonas, silicon dioxide colloidal anhydrous), macrogoal 6000, dye bright red 4R Ponso 4R E 124, dye yellow decline of FCF E 110.
The description
of the Tablet, coated, from salmoncolored till pink-orange color. On a surface of tablets the insignificant marbling is allowed. On cross section two layers are visible.
Pharmacotherapeutic group
Anti-inflammatory and antirheumatic drugs. Non-steroidal anti-inflammatory drugs. Acetic acid derivatives. Diclofenac
ATX M01AB05 Code
Pharmacological
Pharmacokinetics Absorption properties. At intake, diclofenac is quickly and completely absorbed. The concomitant use of food reduces absorption speed, without changing amount of the absorbed active ingredient.
The maximum concentration in blood (Cmax) after single use in diclofenac in a dose of 50 mg is reached approximately in 2 hours and makes 1.5 mkg/ml (5 µmol/l). The amount of the absorbed active ingredient is proportional to a dose.
About a half of a dose of diclofenac is metabolized during the first passing through a liver (effect of the first passing). In case of observance of the recommended interval of dosing of accumulation of drug it is not observed.
Distribution. Communication with proteins of plasma more than 99%. The expressed volume of distribution is from 0.12 to 0.17 l/kg. Diclofenac gets into synovial fluid where reaches the maximum concentration in 3-6 hours. In 2 hours after achievement of peak concentration in blood plasma the levels of active agent in synovial fluid are already higher, than in plasma, and remain higher till 12 o'clock.
Biotransformation. It is metabolized in a liver partially by a glyukuronidation of an initial molecule, but mainly due to single and multiple hydroxylation and a metoksilirovaniye that leads to formation of several phenolic metabolites, the majority of which are converted into glyukuronidny conjugates. Two of these phenolic metabolites are biologically active, but to a lesser extent, than diclofenac.
Elimination. The general system clearance of diclofenac from plasma of 263 ml/min. Elimination half-life (T½) from plasma about 2 hours, from synovial fluid of 3-6 hours. It is removed by kidneys (40-65%), with bile and a stake (35%).
Pharmacokinetics at various groups of patients. Significant differences in absorption, metabolism or removal of drug depending on age of patients are not observed.
At patients with a renal failure the accumulation of not changed active ingredient is not allowed.
At patients with chronic hepatitis or nedekompensirovanny cirrhosis pharmacokinetic parameters of diclofenac same, as well as at the patients who do not have liver diseases.
Pharmacodynamics
Anti-inflammatory, analgetic, febrifuge.
The mechanism of effect of diclofenac is caused by inhibition of cyclooxygenases (TsOG-1 and TsOG-2) therefore reactions of an arachidonic cascade are blocked and synthesis of prostaglandins (PGE2 and PGF2alfa), A2 thromboxane, prostacyclin, leukotrienes and emission of lizosomalny enzymes and also suppression of aggregation of thrombocytes is broken. Diclofenac eliminates or considerably reduces expressiveness of symptoms of inflammation, reduces the hypersensitivity of nerve terminations induced by prostaglandins to biologically active agents which are formed in the inflammation center, lowers the body temperature (preventing effect of prostaglandins on a hypothalamic link of thermal control), reduces concentration of prostaglandins in menstrual blood and intensity of pain at primary dysmenorrhea.
In rheumatic diseases the anti-inflammatory and analgetic properties of diclofenac provide considerable reduction of expressiveness of such symptoms and complaints as a rest pain and at the movement, the morning constraint, a swelling of joints and also improves functions of joints.
At the posttraumatic and postoperative inflammatory phenomena diclofenac quickly stops both spontaneous pain, and an oxycinesia, reduces inflammatory hypostasis and hypostasis of a postoperative wound.
In researches on cellular cultures sodium diclofenac in concentration, equivalent subjects which are reached at treatment of patients does not suppress biosynthesis of proteoglycans of cartilaginous tissue.
Diclofenac shows the significant analgeziruyushchy effect in moderate and severe pain of not rheumatic origin (including at migraine attacks), is capable to eliminate pain and to reduce expressiveness of blood losses at primary dysmenorrhea.
Indications
- inflammatory and degenerative forms of rheumatic diseases (the pseudorheumatism ankylosing a spondylitis, an osteoarthrosis, spondylarthrites), pain syndromes with localization in a backbone, not joint rheumatism
- bad attacks of gout
- the posttraumatic and postoperative pain syndromes which are followed by inflammation and hypostases, for example after dental and orthopedic interventions
- the gynecologic diseases which are followed by a pain syndrome and inflammation, for example primary dysmenorrhea or an adnexitis
- in combination therapy of ENT organs in the serious inflammatory illness which is followed by pain (a pharyngotonsillitis, otitis and others)
Appoint the Route of administration and doses the adult inside in time or at once after a meal, without chewing, washing down with enough water.
The dose is recommended to be selected individually and also to accept the lowest effective dose during shorter time.
For adults the recommended initial daily dose makes 100-150 mg. In mild cases, as well as at long-term therapy, there is usually enough purpose of 75-100 mg.
The general daily dose for adults is usually divided into 2-3 receptions. The daily dose of drug should not exceed 150 mg.
At primary dysmenorrhea the daily dose is selected individually, and usually it makes 50-150 mg. The initial dose can make 50-100 mg, but in case of need it is possible to raise it during several menstrual cycles, but not above than to 200 mg/days. Use of drug should be begun after emergence of the first painful symptoms, duration, depending on symptomatology as soon as possible, makes up to several days.
Single and daily doses and also duration of treatment is established individually by the doctor.
Side effects
Often (from & gt, 1/100 to & lt, 1/10)
- a headache, dizziness
- vertigo
- nausea, vomiting, diarrhea, dyspepsia, abdominal pain, a meteorism, anorexia
- increase in level of transaminases
- rash
Seldom (from & gt, 1/10000 to & lt, 1/1000)
- reactions of hypersensitivity, anaphylactic/anaphylactoid reactions, including hypotension and shock
- drowsiness
- asthma (including dispnoe)
- gastritis, gastrointestinal bleeding (a hematemesis, a melena, diarrhea with blood impurity), ulcer of stomach and intestines, followed or not followed by bleeding or perforation
- hepatitis, jaundice, an abnormal liver function
- urticaria, irritations of skin
Very seldom (& lt, 1/10000)
- thrombocytopenia, a leukopenia, hemolytic anemia, aplastic anemia, an agranulocytosis
- a Quincke's disease
- a disorientation, a depression, insomnia, nightmares, irritability, psychotic disorders
- paresthesia, disturbance of memory, a spasm, alarm, a tremor, aseptic meningitis, a dysgeusia, an acute disorder of cerebral circulation
- a disorder of vision, the obscured sight, a diplopia
- a ring in ears, a hearing disorder
- a cardiopalmus, a stethalgia, heart failure, a myocardial infarction
- hypertensia, a vasculitis
- pneumonia
- colitis (including hemorrhagic colitis and exacerbation of ulcer colitis or Crohn's disease), a constipation, stomatitis, a glossitis, disturbance from a gullet, diafragmopodobny intestinal strictures, pancreatitis
- lightning hepatitis, necrotic hepatitis, a liver failure
- bullous dermatitis, eczema, an erythema, a multiformny erythema, Stephens-Johnson's syndrome, a toxic epidermal necrolysis (Lyell's disease), exfoliative dermatitis, an alopecia, reaction of photosensitivity, purple, purple of Shenleyna-Genokh, an itching
- an acute renal failure, a hamaturia, a proteinuria, a nephrotic syndrome, interstitial nephrite, papillary necrosis
of the Contraindication
- hypersensitivity to diclofenac and any other ingredients of drug
- a peptic ulcer of a stomach or intestines, bleeding or perforation
- hereditary intolerance of fructose, deficiency of Lapp-lactases enzyme, glucose malabsorption - galactoses
- pregnancy and the period of a lactation
- heavy abnormal liver functions, kidneys and heart
- existence in the anamnesis of attacks of asthma, urticaria, acute rhinitis on intake of acetylsalicylic acid or other non-steroidal anti-inflammatory drugs
- diseases of the haematogenic system
- treatment of postoperative pain after operation of coronary shunting or use of the cardiopulmonary bypass
- children's age up to 18 years (because of presence of dyes)
Medicinal interactions
Diclofenac can increase concentration of lithium and digoxin in blood plasma. Monitoring of the last in blood plasma is recommended.
Diclofenac, as well as other non-steroidal anti-inflammatory drugs (NPVP), at combined use with diuretics or antihypertensive drugs (for example - blockers, APF inhibitors) can reduce their antihypertensive effect. Therefore it is necessary to appoint a combination of such drugs with care, and patients (especially advanced age) should control arterial blood pressure periodically. Patients should use enough water, and after the beginning and after the end of the accompanying therapy it is necessary to control periodically function of kidneys, in particular at use of diuretics and APF inhibitors, owing to the increased risk of emergence of nephrotoxicity.
Simultaneous use of kaliysberegayushchy diuretics can lead to increase in level of potassium in blood serum (in case of such combination of medicines this indicator should be controlled often).
Combined use of diclofenac, as well as other system non-steroidal anti-inflammatory drugs, or corticosteroids can increase the frequency of the undesirable phenomena from a digestive tract.
At the patients applying at the same time diclofenac, anticoagulants and antitrombotichesky drugs the increase in risk of bleedings is possible. Therefore in case of such combination of medicines the careful and regular observation of patients is recommended.
Simultaneous use of system non-steroidal anti-inflammatory drugs and selective serotonin reuptake inhibitors can increase risk of gastrointestinal bleeding.
Simultaneous use of diclofenac and antidiabetic drugs is possible, at the same time the efficiency of the last does not change. However in such cases the development of both a hypoglycemia, and a hyperglycemia is very seldom possible that causes need of change of a dose of glucose-lowering drugs during drug use. For this reason it is recommended to control during therapy glucose level in blood.
It is necessary to be careful at diclofenac use less than in 24 hours prior to or after reception of a methotrexate as in such cases the concentration of a methotrexate in blood can increase and amplify its toxic action.
Simultaneous use of diclofenac, a kolestipol or holestiramin reduces diclofenac absorption approximately by 30% and 60% respectively. The drugs should be taken at an interval of several hours.
The drugs stimulating microsomal enzymes of a liver, for example rifampicin, carbamazepine, Phenytoinum, a St. John's wort (Hypericum perforatum) are theoretically capable to reduce concentration of diclofenac in blood plasma.
Influence of diclofenac on synthesis of prostaglandins in kidneys can increase nephrotoxicity of cyclosporine. Therefore it is necessary to appoint drug in smaller doses, than those which would be appointed to the patients who are not accepting cyclosporine.
At the patients applying at the same time antibacterial agents derivative a hinolon and diclofenac the development of spasms is seldom possible.
Special instructions
Impact on digestive tract
At use of all NPVP, including diclofenac, are known cases of development of gastrointestinal bleeding, an ulceration or perforation which can be lethal and arise at any time at patients with existence or lack of the previous symptoms, serious gastrointestinal diseases in the anamnesis. The risk of gastrointestinal bleeding is increased at increase in a dose of NPVP, at patients with an ulcer in the anamnesis, especially, complicated bleeding or perforation and also at elderly patients. For reduction of risk of gastrointestinal toxicity at patients with digestive tract diseases in the anamnesis, stomach cankers, ulcer colitis or Crohn's disease and also at elderly patients, treatment has to be carried out with use of a minimal effective dose of drug and under careful medical observation. The extra care is recommended to be observed at patients who receive at the same time the medicines increasing risk of an ulceration or bleeding such as system corticosteroids, anticoagulants, antithrombocytic means or selective serotonin reuptake inhibitors. For patients with the increased risk of development of gastrointestinal complications and also for patients, receiving therapy by low doses of acetylsalicylic acid, it is necessary to consider the possibility of use of combination therapy of diclofenac, for example, with inhibitors of the protonew pump or mizoprostoly.
In case of development in patients of gastrointestinal bleeding or an ulceration of Ortofen-Zdorovye it is necessary to cancel.
Patients with gastrointestinal disturbances in the anamnesis, especially elderly patients, have to report about any abdominal symptoms (especially about gastrointestinal bleeding).
Patients with respiratory diseases
At patients with asthma, seasonal allergic rhinitis, puffiness of a mucous membrane of a nose, chronic obstructive diseases of lungs or persistent infections of airways (especially if they are connected with symptoms similar to allergic rhinitis) a Quincke's edema or a small tortoiseshell are noted more often in comparison with other patients. To these patients and also patients with an allergy to other drugs (rash, an itching or urticaria), when prescribing the drug Ortofen-Zdorovye it is necessary to observe extra care.
Skin reactions
Very seldom at use of NPVP, including diclofenac, can arise serious skin reactions, including lethal (exfoliative dermatitis, Stephens-Johnson's syndrome and a toxic epidermal necrolysis). Risk of development of such reactions at patients, in most cases, is increased within the first month of treatment. It is necessary to stop use of the drug Ortofen-Zdorovye at appearance of skin rash, injuries of a mucous membrane or other signs of hypersensitivity.
As well as in a case with other NPVP, at drug Ortofen-Zdorovye use the allergic reactions, including anaphylactic/anaphylactoid reactions, can develop in rare instances at patients at whom allergic reactions to diclofenac were not observed earlier.
Impact on a liver
Careful medical observation is necessary when prescribing the drug Ortofen-Zdorovye for patients with an abnormal liver function as their state can worsen.
During long-term treatment the drug Ortofen-Zdorovye, as a precautionary measure, recommends to carry out regular monitoring of function of a liver. As increase in activity of liver enzymes can cause other NPVP, diclofenac. At preservation or further increase in activity of liver enzymes, development of clinical symptoms of disturbance of hepatic function or emergence of other symptoms (for example, eosinophilias, rashes), it is necessary to cancel administration of drug. It must be kept in mind that hepatitis against the background of use of diclofenac can develop without the previous symptoms.
It is necessary to be careful when prescribing the drug Ortofen-Zdorovye to patients with a hepatic porphyria as drug can provoke a porphyria attack.
Impact on kidneys
As cases of a delay of liquid and hypostases at treatment of NPVP, including diclofenac are known, the extra care needs to be observed at patients with dysfunctions of heart or kidneys, with hypertensia in the anamnesis, at elderly patients, patients receiving the accompanying treatment by diuretics or medicines which can make considerable impact on function of kidneys and also at patients with significant reduction of volume of extracellular liquid for any reason to or after big operation. At use of the drug Ortofen-Zdorovye in such cases, as a precautionary measure, it is recommended to carry out monitoring of function of kidneys. Drug phase-out usually leads to restoration of function of kidneys to initial level.
Impact on the cardiovascular
Treatment of NPVP system, including diclofenac, especially in high doses and can be associated for a long time with small increase in risk of development of the serious cardiovascular trombotichesky phenomena (including a myocardial infarction and a stroke). To minimize potential risk of the undesirable cardiovascular phenomena at the patients accepting NPVP, especially in case of existence of factors of cardiovascular risk the smallest effective dose during the shortest possible span has to be used.
Hematologic effects
during long-term treatment diclofenac, as well as other NPVP, recommend to carry out control of all-complete blood count test. As well as other NPVP, diclofenac can temporarily reduce aggregation of thrombocytes. For patients with disturbances of a hemostasis it is necessary to establish careful observation, systematic control of a picture of peripheral blood is necessary.
Geriatrics
elderly patients need to be careful when prescribing drug. In particular, are recommended to appoint the smallest effective dose the weakened elderly patients or patients with low body weight.
Diclofenac, thanks to the pharmakodinamichesky properties, can mask the complaints and symptoms characteristic of infectious and inflammatory diseases.
Pregnancy and the period of a lactation
Use of diclofenac for pregnant women was not studied. Therefore Ortofen-Zdorovye should not appoint throughout the two first trimesters of pregnancy if only the advantage of its use does not exceed risks for a fruit. As well as for other NPVP, use of drug throughout the third trimester of pregnancy is contraindicated owing to risk of development of weakness of patrimonial activity of a uterus and/or premature closing of an arterial channel.
As well as other NPVP, diclofenac in small amounts are allocated in breast milk. Thus, Ortofen-Zdorovye should not be applied during feeding by a breast to prevent undesirable reactions at the child.
As well as other NPVP, diclofenac can negatively affect female fertility therefore the women planning pregnancy are not recommended to appoint drug. At the women having problems with conception or passing a research on infertility it is necessary to consider expediency of drug withdrawal of Ortofen-Zdorovye.
The feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
to the Patients who are feeling dizzy during time reception Ortofena-Zdorovye or other unpleasant feelings from the central nervous system, including disorders of vision, should not drive the car or to operate mechanisms.
Overdose
Symptoms: vomiting, gastrointestinal bleedings, diarrhea, dizziness, a ring in ears or spasms. In case of the profound poisoning the development of an acute renal failure and damages of a liver is possible.
Treatment: gastric lavage, intake of adsorbent. If necessary the supporting and symptomatic therapy which is shown at such complications as arterial hypotension, a renal failure, spasms, disturbances from a digestive tract and respiratory depression. It is improbable that an artificial diuresis, the hemodialysis or hemoperfusion will be useful to diclofenac removal as active agents of non-steroidal anti-inflammatory drugs substantially contact proteins of blood plasma and are exposed to intensive metabolism.
A form of release and packing
On 10 or 30 tablets in blister strip packaging from a film of polyvinylchloride and aluminum foil.
1 blister strip packaging on 30 tablets or 3 blister strip packagings on 10 tablets together with the instruction for medical use in a box of cardboard.
To Store storage conditions in original packing at
a temperature not higher than 25 S. Hranit out of children's reach!
A period of storage
3 years
not to use drug after the expiration date specified on packing!
Prescription status
According to the prescription
LLC Pharmatsevticheskaya Producer the Health company.
Ukraine, 61013, Kharkiv, Shevchenko St., 22.
Owner of the registration certificate
of LLC Pharmatsevticheskaya Health company, Ukraine.
The address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods)
of limited liability partnership of Pharmaceutical Euro
050039, Almaty, Mailing St., 72, apartment 34
Ph.: +7 (727) 271-10-17
Fax: +7 (727) 271-84-97
E-mail:
To develop farmevro@mail.ru