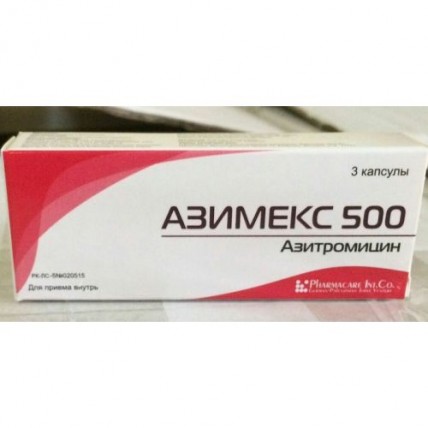
Azimeks 3's 500 mg capsule
- $27.10
Instruction for medical use of AZIMEKS medicine Trade name of Azimex International unlicensed name Azithromycin Dosage Form of the Capsule of 500 mg Structure active agents: azithromycin (in the form of a dihydrate) 500 mg, excipients: starch corn dry, magnesium stearate, structure of the capsule: gelatin, water purified, E 218 methylparaben, E 216 propylparaben, diamond black E 151, red charming E 129, the titan E 171 dioxide. The description the Solid gelatin capsule No. 1 consisting of the body and a lid of burgundy color. Capsule contents – powder of white color. Pharmacotherapeutic group Antimicrobial means for system use. Macroleads, linkozamida and streptogramina. Macroleads. Azithromycin. The ATX J01FA10 code the Pharmacological Pharmacokinetics Later properties of intake azithromycin is quickly soaked up, the bioavailability is about 37%. Time of achievement of the maximum plasma concentration (Cmax) about 2-3 hours, at single dose of azithromycin in a dose of 500 mg of Cmax is 0.4 mg/l. Azithromycin is quickly distributed in fabrics and biological liquids, creating in concentration fabrics, up to 50 times exceeding plasma (the volume of distribution is about 31 l/kg). After single dose of azithromycin in a dose of 500 mg the concentration of drug in tissue of lungs, tonsils, a prostate exceeds MPK90 for expected activators. Due to ability to get and accumulate in the phagocytes migrating in the center of inflammation, concentration of azithromycin in the infected fabrics higher in comparison with not infected (on average for 24-34%). Bactericidal concentration of azithromycin remain in the inflammation center within 5-7 days after reception of the last dose. Extent of linking of azithromycin with serumal proteins varies and depends on plasma concentration, making at 0.05 mg/l about 50% and 12% at 0.5 mg/l. Elimination half-life for azithromycin is in the range from 2 up to 4 days. The main way of elimination (about 59%) – in an invariable view with bile. The rest is exposed to metabolism (N - and About – demethylation, hydroxylation of a dezozamin and aglikonovy ring) with formation of 10 inactive metabolites. With urine 6% of the accepted dose are removed on average. Renal failure: at patients with the profound renal failure (SKF & lt, 10 ml/min.) noted increase in Cmax and AUC 0-120 for 61% and 35% respectively at intake of azithromycin in a dose of 1 g. In a slight and moderate renal failure (SKF from 10 ml/min. to 80 ml/min.) Cmax and AUC 0-120 were increased by 5.1% and 4.2% respectively. Liver failure: at patients with a slight and moderate liver failure changes in serumal concentration of azithromycin in comparison with patients without changes of function of a liver are not revealed that can speak compensatory increase in renal clearance. Elderly patients: at elderly men the pharmacokinetics did not differ from that at men whose age less than 45 years, however at elderly women the increase in plasma concentration for 30-50% without drug cumulation was noted. The pharmacodynamics Azimeksa Active ingredient is azithromycin – a makrolidny antibiotic of group of azaleads. The mechanism of antibacterial action of an aziromitsin is caused by its ability to suppress synthesis of bacterial proteins by linking with a 50S-subunit of a ribosome and inhibition of a stage of a peptide translocation. Azithromycin possesses a wide range of antimicrobic activity concerning gram-positive, gram-negative, intracellular and other microorganisms: Aerobic gram-positive bacteria: Staphylococcus aureus, Streptococcus pneumonia, Streptococcus pyogenes (group A), Streptococcus viridans Aerobic gram-negative bacteria: Haemophilus influenza, Haemophilus parainfluenzae, Haemophilus ducreyi, Legionella pneomophila, Moraxella catarrhalis, Pasteurella multocida, Bordetella pertussis, Bordetella parapertussis, Escherichia coli, Neisseria gonorrhoeae, Helicobacter pylori Anaerobic bacteria: Bacteroides fragilis, Clostridium perfringens, Fusobcterium spp., Prevotella spp., Porphyromonas spp., Propionibacterium spp. Other microorganisms: Chlamidia trachomatis, Chlamidia pneumonia, Mycoplasma pneumonia, Mycoplasma hominis, Borrelia burgdorferi, Treponema pallidum, Ureaplsma ureolyticum. Azithromycin is not active concerning the gram-positive bacteria resistant to erythromycin. Indications of Azimex it is applied to treatment of the infectious and inflammatory diseases caused by activators, sensitive to drug: an upper respiratory tract infection and ENT organs (bacterial pharyngitis / tonsillitis, sinusitis, average otitis) and the lower airways (bronchitis, bacterial and atypical pneumonia) of an infection of skin and soft tissues (the ugly face, impetigo which are again infected a dermatosis, the chronic migrating erythema – an initial stage of a disease of Lyme) infections of an urogenital path (uncomplicated urethritis, and/or the cervicitis caused by Neisseria gonorrhoeae, Chlamydia trachomatis) the diseases of a stomach and a duodenum associated with Helicobacter pylori. The route of administration and doses the Mode of dosing is set individually depending on age, body weight, function of kidneys and severity of an infection. As the concomitant use of food slows down absorption of azithromycin, Azimex needs to accept in 1 hour prior to meal or in 2 hours after meal. To children aged from 6 months is also more senior it is recommended to appoint drug in the form of suspension. In an infection of upper and lower airways, skin and soft tissues (except for the chronic migrating erythema) Adults: on 500 mg of 1 times a day within 3 days or 500 mg a day in the first day and on 250 mg a day for the next 4 days. Course dose of 1500 mg. Children: appoint at the rate of 10 mg/kg of body weight of 1 times a day within 3 days or in the first day of 10 mg/kg and then 5 mg/kg for the next 4 days. Course dose of 30 mg/kg. In uncomplicated clamidiosis: 1 g (1000 mg) once. In an uncomplicated gonorrheal urethritis and a cervicitis: 2 g (2000 mg) once. In Lyme's disease (borreliosis) for treatment of an initial stage (erythema migrans) Adult: on 1 g (2 caps on 500 mg or 4 caps. on 250 mg) in the 1st day and on 500 mg daily from the 2nd to the 5th day (a course dose – 3 g). Children: Reception of capsules In the diseases of a stomach and duodenum associated with Helicobacter pylori is not recommended to children up to 12 years: on 1 g (2 caps. on 500 mg) in day within 3 days as a part of combination therapy. Side reactions Often (& gt, 1/100, & lt, 1/10) - nausea, vomiting, diarrhea, an abdominal pain not often (& gt, 1/1000, & lt, 1/100) - a liquid chair, a meteorism, a digestive disturbance, loss of appetite Seldom (& gt, 1/10000, & lt, 1/1000) - a headache, dizziness, drowsiness, spasms, a dysgeusia - a tromotsitopeniya - aggression, excitement, concern, nervousness, insomnia - paresthesia and an asthenia - a hearing disorder, deafness and sonitus - tachycardia, arrhythmia with ventricular tachycardia, lengthening of an interval of QT-feasts, the arrhythmia which is followed by development as torsade de pointes. - discoloration of language, a constipation, pseudomembranous colitis - tranzitorny rise in level aminotrasferaz a liver, bilirubin, cholestatic jaundice, hepatitis - reactions of hypersensitivity (reddenings, skin rash, an itching, a small tortoiseshell, a Quincke's disease, photosensitivity), a multiformny erythema, Stephen-Johnson's syndrome and a toxic epidralny necrolysis - an arthralgia Very seldom (& gt, 1/10000, & lt, 1/1000) - interstitial nephrite, an acute renal failure - fatigue, convulsions - change of taste and sense of smell - vaginita, candidiasis, superinfections - the acute anaphylaxis, including swelled (in rare instances leading to death) Contraindications: - hypersensitivity to antibiotics of group of macroleads - heavy renal failures and a liver - pregnancy and the period of a lactation - children's age up to 12 years With care: - in arrhythmia (ventricular arrhythmias, lengthening of an interval of QT are possible) - to children, with the profound abnormal liver functions and kidneys Medicinal interactions Antiacid drugs: at a concomitant use the decrease in level of the maximum plasma concentration of azithromycin by 30% in the absence of influence on the general bioavailability is possible. It is recommended to accept azithromycin in 1 hour prior to or in 2 hours after reception of antacids. Oral anticoagulants: increase in risk of bleeding at a concomitant use with warfarin and other derivatives of coumarin. It is necessary to be careful, periodically to control fibrillation parameters. Astemizol, to triazoles, midazolam, alfentanil: it is necessary to be careful at simultaneous use with azithromycin in view of the described interaction of erythromycin with these medicines with increase in their concentration and the rendered therapeutic effect. Digoxin: at a concomitant use the increase in plasma concentration of digoxin in view of ability of some makrolidny antibiotics to suppress his metabolism is possible, monitoring of plasma concentration of digoxin is recommended. Zidovudine: single 1000 mg a dose of azithromycin and repeated doses in 1200 mg or 600 mg did not affect plasma concentration and renal excretion of a zidovudine or its glucuronides. However prescribing of azithromycin increases concentration of an active metabolite of a zidovudine in peripheral blood mononuclear cells. The clinical value of interaction is unknown, but it can have positive therapeutic value. Carbamazepine: in pharmacokinetic researches on volunteers the change of plasma concentration of carbamazepine or its active metabolites was not revealed. Nelfinavir: in a research on healthy volunteers and a nelfinavira at achievement of stable concentration at reception in a dose of 750 mg 3 times a day led co-administration of azithromycin in a dose of 1200 mg to 100% to increase in absorption and bioavailability of azithromycin. Clinical consequences of this interaction are unknown, it is necessary to be careful when prescribing azithromycin to the patients accepting nelfinavir. Ergot derivatives: there is a theoretical possibility of development of an ergotism, co-administration is not recommended. Rifabutin: co-administration does not change serumal concentration of drugs. At co-administration, cases of a neutropenia which, however, can be caused by reception of each of these drugs were revealed. Theophylline: increase in plasma concentration of theophylline is possible, it is necessary to be careful at co-administration. Terfenadin: pharmacokinetic researches did not reveal pharmacokinetic interaction at co-administration. However, in view of the described reactions of interaction of a terfenadin it is necessary to be careful with other makrolidny antibiotics which were followed by increase in an interval of QT at co-administration. Cyclosporine: in a pharmacokinetic research on healthy volunteers the three-day intake of azithromycin in a dose of 500 mg/day and cyclosporine in a dose of 10 mg/kg led to significant increase in Smaks and AUG0-5 by 24% and 21% respectively. At co-administration it is necessary to be careful and monitorirovat plasma concentration of cyclosporine. Special indications of Reaction of hypersensitivity As well as at reception of other makrolidny antibiotics, when prescribing azithromycin it was reported about exceptional cases of development of serious allergic reactions, including a Quincke's disease and anaphylactic reactions (in rare instances with a lethal outcome). Resuming of symptomatology was characteristic of some of these reactions that demanded longer observation and treatment. Lengthening of an interval of QT For antibiotics of group of macroleads characteristic is ability to extend time of repolarization and an interval of QT that is risk factor of developing arrhythmia and torsade de pointes. The possibility of similar effect cannot be completely excluded also for azithromycin at the patients having the increased risk of lengthening of time of repolarization (patients with the inborn or acquired lengthening of an interval of QT, patients with disturbances of water and electrolytic balance, for example a hypopotassemia or hypomagnesiemia, patients with bradycardia, arrhythmia or heavy heart failure, the patients who are at the same time taking other drugs which can cause lengthening of an interval of QT, for example the antiarrhytmic drugs IA and III of classes, tsizaprid or terfenadin). These patients are not recommended to appoint azithromycin. The risk of superinfection As well as for other antibiotics of a broad spectrum of activity, against the background of intake of azithromycin exists risk of development of the superinfection caused by insensitive microorganisms (for example, fungal infections or pseudomembranous colitis). Control of a condition of the patient of rather possible signs of development of superinfection is recommended. Use in a renal failure with a moderate renal failure (SKF of 10 - 80 ml/min.) is not required From patients correction of the mode of dosing. It is necessary to be careful when assigning to patients with heavy renal failures (SKF ˂ 10ml/mines) as at the same time in 33% of cases the increase in system influence is noted. Use in an abnormal liver function As azithromycin is exposed to intensive metabolism in a liver and is excreted with bile, drug should not be appointed to patients with heavy abnormal liver functions. Researches on assessment of use of azithromycin for this category of patients were not conducted. At development of abnormal liver functions, drug should be cancelled. To patients with neurologic or psychiatric disturbances azithromycin has to be appointed with care. Azithromycin is not appointed for treatment of the infected burn wounds. The oral form of azithromycin should not be appointed in the heavy infections demanding fast achievement of high plasma concentrations. Features of influence of medicine ability to run the vehicle or potentially dangerous mechanisms Special researches on definition of potential impact of azithromycin on ability to driving of motor transport and work with dangerous mechanisms were not carried out. However at implementation of these types of activity requiring special attention it is necessary to take ability of azithromycin to cause dizziness and spasms into account. Overdose Symptoms: reversible hearing loss, profound nausea, severe vomiting and diarrhea. Treatment: gastric lavage and symptomatic therapy. Form of release and packing of the Capsule of 500 mg No. 3. On 3 capsules place in blister strip packaging from a film of polyvinylchloride and aluminum foil. On 1 blister strip packaging together with the instruction for medical use in the state and Russian languages place in a cardboard pack. To Store storage conditions in the dry, protected from light place, at a temperature not above 25 °C. To store out of children's reach. A period of storage 3 years Prescription status According to the prescription the Producer of Birzeyt, Palestine Industrial zone 79, Ramallah ph. + 970-2-2987574 Owner of the registration certificate German-Palestinian joint venture Pharmakar Int. To., Palestine Ramallah, Betuniya, the Industrial zone, p/o, p.o. box 51621. The address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of this product of El company LLP, Almaty, Masangchi St. 98 A, office 41
to Develop
to Develop