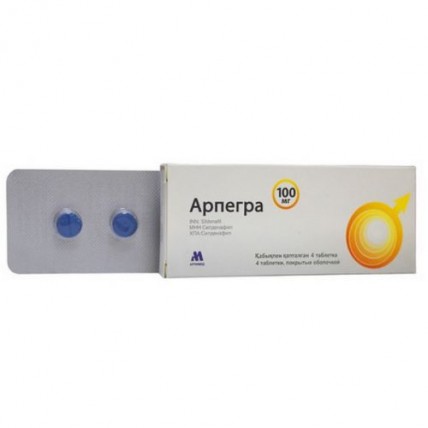
Arpegra (Sildenafil) 100 mg 4 coated tabs
- $12.70
Indications for use
- erectile dysfunction, characterized by an inability to achieve or maintain an erection of the penis sufficient for satisfactory intercourse.
The drug is effective only for sexual stimulation.
List of information required prior to use
Contraindications
- hypersensitivity to the active substance or to any of the excipients
- simultaneous administration of drugs that are donors of nitric oxide
(amyl nitrite) or nitrates in any form, since sildenafil enhances the hypotensive effect of nitrates, due to the known effect on the nitric oxide / cyclic guanosine monophosphate pathway
- the simultaneous use of PDE-5 inhibitors, such as sildenafil with guanylate cyclase stimulants (riociguat), is contraindicated, as this can lead to symptomatic hypotension
- patients for whom excessive sexual activity is not recommended (patients with severe cardiovascular diseases, such as unstable angina pectoris or acute heart failure)
- loss of vision in one eye due to anterior non-arteritis ischemic neuropathy of the optic nerve (NPINZN), regardless of whether this episode was associated with a previous use of a PDE-5 inhibitor or not
- according to a registered indication, the drug is not intended for use in children and adolescents under the age of 18 and in women.
There are no controlled clinical studies of the safety and efficacy of sildenafil and therefore the use of the drug is contraindicated in the following groups:
- severe hepatic impairment
- arterial hypotension (blood pressure <90/50 mm Hg)
- myocardial infarction suffered in the last 6 months
- a stroke suffered in the last 6 months
- hereditary degenerative diseases of the retina (for example, hereditary retinitis pigmentosa in a small number of these patients have hereditary dysfunctions of retinal phosphodiesterases). Necessary precautions for use
To diagnose erectile dysfunction and determine its possible underlying causes, medical history and physical examination should be carried out before pharmacological treatment is considered.
Risk factors for cardiovascular disease
Before starting treatment for erectile dysfunction, the attending physician should assess the cardiovascular status of their patients, since there is a certain degree of risk of heart complications associated with sexual activity.
Sildenafil has vasodilating properties, resulting in a mild or transient decrease in blood pressure.
Before prescribing sildenafil, physicians should carefully check the likelihood of undesirable effects of the vasodilating effect of this drug on the condition of patients with certain underlying diseases, especially in their combination with sexual activity. The group of hypersensitivity to vasodilators includes patients with narrowing of the left ventricular outlet (for example, aortic valve stenosis, hypertrophic obstructive cardiomyopathy), as well as patients with a rare syndrome of multiple systemic atrophy, manifested as a severe degree of impairment of autonomous blood pressure control.
The drug enhances the hypotensive effect of nitrates.
During the post-registration period, cases of serious cardiovascular complications have been reported, including myocardial infarction, unstable angina pectoris, sudden cardiac death, ventricular arrhythmia, cerebrovascular bleeding, transient ischemic attack, arterial hypertension and arterial hypotension during the use of sildenafil. Most, but not all, of these patients had antecedent cardiovascular risk factors. Many of these events have been reported during or shortly after the completion of intercourse, and several have been reported shortly after taking sildenafil without sexual activity. It is not possible to determine whether these phenomena were directly related to the use of sildenafil, with sexual activity, with existing cardiovascular disease, with a combination of these factors or with other factors.
Priapism
Medicines for the treatment of erectile dysfunction, including sildenafil, should be used with caution in patients with anatomical deformity of the penis (such as angulation, cavernous fibrosis, or Peyronie's disease) and in patients with conditions that predispose to priapism (such as sickle -cellular anemia, multiple myeloma or leukemia).
Cases of prolonged erection and priapism have been reported during the post-marketing period of sildenafil. In the event of an erection lasting more than 4 hours, the patient should seek urgent medical attention. If left untreated, priapism can damage the tissues of the penis and cause irreversible loss of potency.
Concomitant use with other PDE-5 inhibitors or others drugs for the treatment of erectile dysfunction
The safety and efficacy of the combination of sildenafil with other PDE-5 inhibitors, drugs for the treatment of pulmonary arterial hypertension containing sildenafil, or other drugs for the treatment of erectile dysfunction have not been studied, therefore, the simultaneous use of these combinations is not recommended.
Effects on vision
There have been cases of visual impairment in connection with the intake of sildenafil and other PDE-5 inhibitors, namely, a rare disease - anterior non-arteritis ischemic neuropathy of the optic nerve. In case of any defects in visual acuity, you must stop taking the drug and immediately consult a doctor.
Concomitant use with ritonavir
It is not recommended to take ritonavir with sildenafil.
Simultaneous use with alpha-blockers
With the simultaneous use of sildenafil with alpha-blockers, caution should be exercised, as it can lead to the development of symptomatic arterial hypotension in a group of especially sensitive patients. Its development is most likely in the first 4 hours after taking drugs containing sildenafil. In order to reduce the risk of developing symptomatic arterial hypotension in patients undergoing treatment with alpha-blockers, the hemodynamic state in patients receiving alpha-blockers should be stabilized before starting treatment with sildenafil. In addition, the use of sildenafil should be considered starting with a dose of 25 mg. Patients must be counseled about the measures to be taken in the event of symptomatic postural arterial hypotension.
Effect on blood clotting
In vitro studies using human platelets indicate that sildenafil enhances the antiaggregatory effect of sodium nitroprusside. There are no data on the safety of using sildenafil in patients with bleeding disorders or active peptic ulcers, so sildenafil should be used after a careful assessment of the benefit / risk ratio.
Interaction with other medicinal products
The effect of other drugs on the pharmacokinetics of sildenafil.
In vitro studies
Sildenafil metabolism is mainly mediated by isoforms 3A4 (major pathway) and 2C9 (minor pathway) of cytochrome P450 (CYP). For this reason, inhibitors of these isoenzymes can reduce, and inducers of these isoenzymes can increase the clearance of sildenafil.
In vivo studies
With the simultaneous administration of sildenafil with CYP3A4 inhibitors (such as ketoconazole, erythromycin and cimetidine), a decrease in the clearance of sildenafil is observed. In this group of patients, there is no increased frequency of side effects; nevertheless, it is necessary to start treatment with Arpegra at an initial dose of 25 mg.
Concomitant administration of the HIV protease inhibitor ritonavir, which is a strong inhibitor of cytochrome P450, in an equilibrium state (500 mg twice a day) with sildenafil (100 mg single dose) leads to an increase in the maximum concentration (Cmax) of sildenafil by 300% (4-fold), as well as an increase in the AUC of sildenafil in blood plasma by 1000% (11-fold). After 24 hours, plasma levels of sildenafil are approximately 200 ng / ml, compared to approximately 5 ng / ml after sildenafil alone. These data are consistent with the pronounced effects of ritonavir on a wide range of cytochrome P450 substrates. Based on the above data, concomitant use of sildenafil with ritonavir is not recommended.
Sildenafil does not affect the pharmacokinetics of ritonavir. The maximum dose of sildenafil should under no circumstances exceed 25 mg in 48 hours.
Concomitant administration of the HIV protease inhibitor saquinavir, which is a CYP3A4 inhibitor, in equilibrium (1200 mg three times a day) with sildenafil (100 mg single dose) leads to an increase in the Cmax of sildenafil by 140%, as well as an increase in the AUC of sildenafil by 210%. Sildenafil does not affect the pharmacokinetics of saquinavir. Stronger CYP3A4 inhibitors such as ketoconazole and itraconazole are more effective.
With a single dose of sildenafil 100 mg together with erythromycin, a moderate inhibitor of CYP3A4, in an equilibrium state (500 mg twice a day for 5 days), there is an increase in systemic exposure to sildenafil by 182% (determined by the AUC value).
Azithromycin (at a dose of 500 mg per day for 3 days) does not affect the AUC, Cmax, Tmax, elimination rate constant or the subsequent half-life of sildenafil or its main circulating metabolite.
The simultaneous use of cimetidine (800 mg), which is an inhibitor of cytochrome P450 and a nonspecific inhibitor of CYP3A4 with sildenafil (at a dose of 50 mg), causes an increase in the concentration of sildenafil in blood plasma by 56%.
Grapefruit juice is a weak inhibitor of CYP3A4-mediated metabolism in the intestinal wall and may cause a moderate increase in plasma levels of sildenafil.
One repeated administration of an antacid (magnesium hydroxide / aluminum hydroxide) does not affect the bioavailability of sildenafil.
CYP2C9 inhibitors (tolbutamide, warfarin and phenytoin), CYP2D6 inhibitors (selective serotonin reuptake inhibitors, tricyclic antidepressants), thiazide and thiazide-like diuretics, loop and potassium-sparing diuretics, inhibitors of inhibitors of angioplasty receptors and inducers of the metabolic activity of CYP450 (rifampicin, barbiturates) do not affect the pharmacokinetics of sildenafil.
The simultaneous use of the endothelin antagonist bosentan (a moderate inducer of CYP3A4, CYP2C9 and, probably, CYP2C19) in an equilibrium state (125 mg twice a day) with sildenafil in an equilibrium state (80 mg three times a day) leads to a decrease in AUC and Cmax values for sildenafil by 62.6% and 55.4%, respectively. Thus, it is believed that the simultaneous use with strong inducers of CYP3A4, such as rifampin, causes a more pronounced decrease in the concentration of sildenafil in blood plasma.
Nicorandil is a hybrid of a potassium channel activator and nitrate. Due to the nitrate component, it is potentially capable of entering into serious interactions with sildenafil.
Effect of sildenafil on other medicinal products
In vitro studies
Sildenafil is a weak inhibitor of cytochrome P450 isoforms 1A2, 2C9, 2C19, 2D6, 2E1 and 3A4 (IC50> 150 μM). Provided that after taking the recommended doses of the drug, the maximum plasma concentration of sildenafil is approximately 1 μM, it is unlikely that sildenafil will change the clearance of substrates of these isoenzymes.
There are no data on the interaction of sildenafil with nonspecific phosphodiesterase inhibitors such as theophylline or dipyridamole.
In vivo studies
In accordance with the known effect of sildenafil on the NO / cGMP signaling pathway, sildenafil is capable of enhancing the hypotensive effect of nitrates, namely, a significant decrease in blood pressure. Therefore, its simultaneous use with nitric oxide donors (amyl nitrite) or nitrates in any form is contraindicated.
Rioziguat
In clinical trials, riociguat increased the hypotensive effect of PDE-5 inhibitors. There were no data on a favorable clinical effect when using this combination in the studied population. The simultaneous use of riociguat with PDE-5 inhibitors, including sildenafil, is contraindicated.
Simultaneous reception with alpha-blockers
It is recommended to use sildenafil with caution in patients taking the drug of the alpha-blocker group, since their simultaneous use can lead to symptomatic arterial hypotension in some sensitive patients. This can most likely occur within 4 hours after taking a dose of sildenafil. To reduce the risk of postural arterial hypotension, a state of hemodynamic stability should be achieved in patients receiving alpha-blocker therapy before starting treatment with sildenafil. Consideration should be given to the use of sildenafil starting with a dose of 25 mg. In addition, physicians should instruct patients on how to deal with symptoms of postural hypotension.
With the simultaneous use of sildenafil and doxazosin in patients stabilized with doxazosin therapy, rare cases of symptomatic arterial hypotension, dizziness and lightheadedness, but not fainting, have been reported.
With the simultaneous use of sildenafil (at a dose of 50 mg) with tolbutamide (at a dose of 250 mg) or warfarin (at a dose of 40 mg), both of which are metabolized by CYP2C9, no significant interactions were found.
Sildenafil (at a dose of 50 mg) does not increase the bleeding time caused by the intake of acetylsalicylic acid (at a dose of 150 mg).
Sildenafil (50 mg dose) does not enhance the hypotensive effect of alcohol with an average maximum blood alcohol level of 80 mg / dL.
The combination of the following classes of antihypertensive drugs: diuretics, beta-blockers, ACE inhibitors, angiotensin II antagonists, antihypertensive drugs (vasodilator and central action), adrenergic neuroblockers, calcium channel blockers and blockers of alpha-adrenergic receptors showed no differences in the profile taking sildenafil compared with patients receiving placebo. In special drug interaction studies, during which sildenafil (at a dose of 100 mg) was used simultaneously with amlodipine in patients with arterial hypertension, an additional reduction in systolic blood pressure in the supine position was 8 mm. rt. Art. The corresponding additional reduction in diastolic blood pressure in the supine position was 7 mm. rt. Art. These additional reductions in blood pressure were similar in magnitude to that and that have been observed with sildenafil as monotherapy in healthy volunteers.
Sildenafil (100 mg) does not affect the equilibrium pharmacokinetics of HIV protease inhibitors, saquinavir and ritonavir, both of which are CYP3A4 substrates.
When used simultaneously with bosentan, sildenafil in an equilibrium state (80 mg three times a day) causes an increase in the AUC and Cmax of bosentan (125 mg twice a day) by 49.8% and 42%, respectively, in male patients.
Special warnings
Among women
The drug Arpegra is contraindicated for use in women.
Arpegra should not be used in men with rare hereditary galactose intolerance, Lapp lactase deficiency or glucose and galactose malabsorption syndrome, due to the lactose content in the drug.
Application in pediatrics
The drug is contraindicated for use in children and adolescents under the age of 18 years.
During pregnancy and lactation
Arpegra is not indicated for use by women.
There have been no proper and strictly controlled studies of the use of Arpegra in pregnant and breastfeeding women.
Features of the effect of the drug on the ability to drive vehicles or potentially dangerous mechanisms
Arpegra may have little effect on the ability to drive and operate machinery.
Since dizziness and visual impairment have been reported in clinical trials with sildenafil, patients should know how they react to Arpegra before driving or operating machinery.
Recommendations for use
Dosage regimen
Adult use
The recommended dose is 50 mg, the drug is taken if necessary about 1 hour before intercourse. Taking into account the efficacy and tolerability, the dose can be increased to 100 mg or reduced to 25 mg.
The maximum recommended dose is 100 mg.
The maximum recommended frequency of administration is 1 time per day.
Special patient groups
Elderly patients
In elderly patients (≥ 65 years), dose adjustment is not required.
Patients with impaired renal function
With mild to moderate renal dysfunction (creatinine clearance 30-80 ml / min), dose adjustment is not required. Due to the decrease in sildenafil clearance in patients with severe renal impairment (creatinine clearance <30 ml / min), the use of the drug at a dose of 25 mg should be considered. Based on the effectiveness and tolerability of the drug, if necessary, its dose can be increased in stages up to 50 mg and up to 100 mg.
Patients with impaired liver function
Due to the fact that the clearance of sildenafil is reduced in patients with impaired liver function (for example, cirrhosis), the use of the drug at a dose of 25 mg should be considered. Based on the effectiveness and tolerability of the drug, if necessary, its dose can be increased in stages up to 50 mg and up to 100 mg.
Pediatric and adolescent patients
Sildenafil is not indicated for use in children (<18 years of age).
Use in patients taking other medicines
With the exception of ritonavir, which is not recommended for concomitant use with sildenafil, an initial dose of 25 mg should be considered in patients receiving concomitant therapy with CYP3A4 inhibitors.
In order to reduce the risk of postural arterial hypotension in patients treated with alpha-blockers, the condition of patients receiving alpha-blockers should be stabilized before starting treatment with sildenafil. In addition, the use of sildenafil should be considered starting with a dose of 25 mg.
Method and route of administration
The tablet, if necessary, should be halved.
The tablet must be swallowed whole, without chewing, with a glass of water.
Frequency of application with indication of time of reception
The drug can be taken with or without food. However, it may take longer for the drug to take effect if taken with food.
Measures to be taken in case of overdose
In studies of volunteers using a single dose of up to 800 mg, adverse reactions were similar to those observed at lower doses, but the frequency and severity were higher. Doses of 200 mg did not lead to an increase in efficacy, but the incidence of adverse reactions (headache, flushing, dizziness, dyspepsia, nasal congestion, altered vision) increased.
In cases of overdose, standard supportive treatment should be prescribed. It is assumed that renal dialysis does not accelerate excretion, since sildenafil has a high degree of binding to plasma proteins and is not excreted in the urine.
Seek the advice of a healthcare professional to explain how the drug is used.
Description of undesirable reactions that manifest themselves with the standard use of the drug and the measures to be taken in this case
Determination of the frequency of side effects is carried out in accordance with the following criteria and: very often (≥ 1/10), often (≥ 1/100 to <1/10), infrequently (≥ 1/1000 to <1/100), rarely (≥ 1/10000 to <1/1000 ), very rare (<1/10000), unknown (cannot be estimated from the available data).
Often
- headache
Often
- dizziness
- color distortion of vision (chloropsia, chromatopsia, cyanopsia, erythropsia, xanthopsia), visual impairment, blurred vision
- hyperemia, hot flashes
- nasal congestion
- nausea, dyspepsia
Infrequently
- rhinitis
- hypersensitivity
- drowsiness, hypesthesia
- disorders associated with lacrimation (dry eyes, dysfunction of the lacrimal gland, increased lacrimation), eye pain, photophobia, photopsia, hyperemia of the eye vessels, brightness of visual perception, conjunctivitis
- spatial disorientation (vertigo), tinnitus
- tachycardia, palpitations
- arterial hypertension, arterial hypotension
- nosebleeds, congestion of the paranasal sinuses
- gastroesophageal reflux disease, vomiting, pain in the upper abdomen, dry mouth
- rash
- myalgia, pain in the limbs
- hematuria
- chest pain, increased fatigue, feeling of heat
- increased heart rate
Rarely
- acute cerebrovascular accident, transient ischemic attack, convulsive attack *, recurrence of convulsive attack *, fainting
- anterior non-arteritic ischemic neuropathy of the optic nerve *, retinal vascular occlusion *, retinal hemorrhage, arteriosclerotic retinopathy, retinal disease, glaucoma, visual field defect, diplopia, decreased visual acuity, myopia, asthenopia, floating opacities of the vitreous membrane, disease of the iris , iridescent circles in the field of vision, eye swelling, eye swelling, visual disturbances, conjunctival hyperemia, eye irritation, unusual sensation in the eye, eyelid edema, discoloration of the sclera
- deafness
- sudden cardiac death *, myocardial infarction, ventricular arrhythmia *, atrial fibrillation, unstable angina
- a feeling of tightness in the throat, swelling of the nose, dryness of the nasal mucosa
- oral hypesthesia
- Stevens-Johnson syndrome *, toxic epidermal necrolysis *
- bleeding from the penis, priapism *, hematospermia, increased erection
- irritability
Additional information
Composition of the medicinal product
One tablet contains the active ingredient - sildenafil citrate 146 mg equivalent to sildenafil 100 mg,
excipients: microcrystalline cellulose, lactose monohydrate, calcium hydrogen phosphate, povidone, magnesium stearate, purified talc, sodium starch glycolate,
shell composition: titanium dioxide E171, hypromellose, purified talc, propylene glycol, brilliant blue E133.
Description of appearance, smell, taste
Tablets of a round shape, with a biconvex surface, coated with a blue film with a line, with a diameter of 10.0 mm ± 2% and a thickness of 3.0 to 4.5 mm.
Release form and packaging
4 tablets are placed in a blister strip made of polyvinyl chloride film and aluminum foil.
1 contour package, together with instructions for medical use are placed in a cardboard box.
Shelf life is 3 years.
Do not use after the expiration date!
Storage conditions
Store in a dry, dark place at a temperature not exceeding 25 ° C.
Keep out of the reach of children!