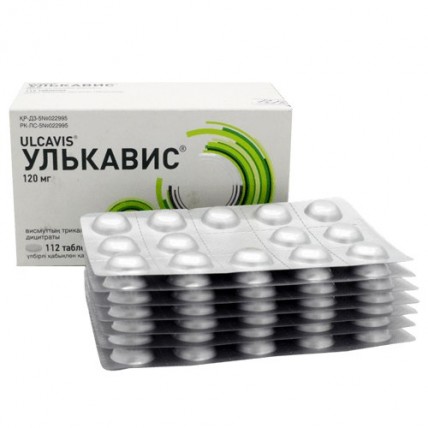
ULCAVIS® (Bismuth Tripotassium Dicitrate) 120 mg, 112 tablets
- $49.00
Sku:
74a82415ae16
Ingredient:
Bismuth Tripotassium Dicitrate
One tablet contains
Absorption
Ulcavis® is a surface-active agent, negligible amounts of bismuth derivatives of bismuth subcitrate are absorbed during treatment (less than 0.2% of the dose).
Distribution
Bismuth is mainly deposited in the kidneys, other organs also contain traces of bismuth.
Biotransformation
Ulcavis is deposited in the stomach into insoluble bismuth complexes, possibly bismuth oxychloride and bismuth citrate.
Selection
The main part of bismuth taken orally is excreted in the feces. Urinary clearance with a small amount of absorbed bismuth is approximately 50 ml/min. However, most of the absorbed bismuth is excreted during a half-life of 5-11 days.
Ulcavis® is an antiulcer agent with bactericidal activity against Helicobacter pylori. It also has anti-inflammatory and astringent properties. In the acidic environment of the stomach, insoluble bismuth oxychloride and citrate are precipitated, chelate compounds with a protein substrate are formed in the form of a protective film on the surface of ulcers and erosions. By increasing the synthesis of prostaglandins E, the formation of mucus and the secretion of bicarbonate, it stimulates the activity of cytoprotective mechanisms, increases the resistance of the mucous membrane of the gastrointestinal tract to the effects of pepsin, hydrochloric acid, enzymes and bile salts. Leads to the accumulation of epidermal growth factor in the area of the defect. Reduces the activity of pepsin and pepsinogen.
inside
Ulcavis® adults are prescribed 1 tablet 4 times a day 30 minutes before meals and at night, or 2 tablets 2 times a day 30 minutes before meals.
Tablets should be taken 30 minutes before meals with a small amount of water.
The duration of the course of treatment is 4-8 weeks.
For the next 8 weeks, preparations containing bismuth should not be used.
For the eradication of Helicobacter pylori, it is advisable to use Ulcavis® in combination with other antibacterial agents with anti-Helicobacter pylori activity and proton pump inhibitors, the so-called quadruple therapy.
Very common (≥ 1/10)
-staining of feces in a dark color due to the formation of bismuth sulfide, but it can be easily distinguished from melena
Uncommon (≥ 1/1000, <1/100)
- nausea, vomiting, diarrhea or constipation
- allergic reactions: skin rash, itching
Very rare (<1/10000)
- anaphylactic reactions
- with prolonged use in high doses - encephalopathy associated with the accumulation of bismuth in the central nervous system
Within half an hour after taking Ulcavis®, it is not recommended to use other medicines orally, as well as the intake of food and liquids, in particular, antacids, milk, fruits and fruit juices. This is due to the fact that they, when taken orally, can affect the effectiveness of Ulcavis®.
Ulcavis® reduces the absorption of tetracycline.
The drug should not be used for more than 8 weeks, it is also not recommended to exceed the established daily doses for adults during treatment. During the period of drug treatment, other drugs containing bismuth should not be used, as the risk of side effects increases. At the end of the course treatment with the drug in recommended doses, the concentration of the active active substance in the blood plasma does not exceed 3-58 μg / l, and intoxication is observed only at a concentration above 100 mg / l.
Prolonged use of large doses of bismuth-containing compounds is not recommended, since in some cases this can lead to reversible encephalopathy. The risk of encephalopathy, however, is minimal when Ulcavis is used at the recommended doses.
When using Ulcavis, staining of feces in a dark color is possible due to the formation of bismuth sulfide. Sometimes there is a darkening of the tongue. It is not recommended to drink alcohol during therapy with Ulcavis.
Features of the effect of the drug on the ability to drive a vehicle or potentially dangerous mechanisms
Does not affect.
Symptoms: dyspepsia, rash, inflammation of the mucous membranes of the mouth, characteristic darkening in the form of blue lines on the gums, impaired renal function.
Treatment: drug withdrawal, gastric lavage, activated charcoal, saline laxatives. The control of kidney function, the concentration of bismuth in the blood and urine is shown. In the future, symptomatic therapy is carried out. In case of impaired renal function, accompanied by a high level of bismuth in the blood plasma, it is possible to introduce complexing agents - dimercaptosuccinic and dimercaptopropanesulfonic acids. In severe renal failure, hemodialysis is indicated.
Store in original packaging at a temperature not exceeding 25 ºС.
Keep out of the reach of children!
Shelf life - 2 years
Do not use after the expiration date
- Active ingredient: Bismuth tripotassium dicitrate 303.03 mg (equivalent to bismuth oxide 120 mg),
- Excipients: corn starch, povidone K-30, potassium polyacrylin, macrogol 6000, magnesium stearate,
- Sheath: Opadry II clear (composition: polyvinyl alcohol, macrogol 4000, talc), titanium dioxide (E171)
Pharmacological properties
Pharmacokinetics
Absorption
Ulcavis® is a surface-active agent, negligible amounts of bismuth derivatives of bismuth subcitrate are absorbed during treatment (less than 0.2% of the dose).
Distribution
Bismuth is mainly deposited in the kidneys, other organs also contain traces of bismuth.
Biotransformation
Ulcavis is deposited in the stomach into insoluble bismuth complexes, possibly bismuth oxychloride and bismuth citrate.
Selection
The main part of bismuth taken orally is excreted in the feces. Urinary clearance with a small amount of absorbed bismuth is approximately 50 ml/min. However, most of the absorbed bismuth is excreted during a half-life of 5-11 days.
Pharmacodynamics
Ulcavis® is an antiulcer agent with bactericidal activity against Helicobacter pylori. It also has anti-inflammatory and astringent properties. In the acidic environment of the stomach, insoluble bismuth oxychloride and citrate are precipitated, chelate compounds with a protein substrate are formed in the form of a protective film on the surface of ulcers and erosions. By increasing the synthesis of prostaglandins E, the formation of mucus and the secretion of bicarbonate, it stimulates the activity of cytoprotective mechanisms, increases the resistance of the mucous membrane of the gastrointestinal tract to the effects of pepsin, hydrochloric acid, enzymes and bile salts. Leads to the accumulation of epidermal growth factor in the area of the defect. Reduces the activity of pepsin and pepsinogen.
Indications for use
- Peptic ulcer of the stomach and duodenum
- Chronic gastritis and gastroduodenitis in the acute phase, including those associated with Helicobacter pylori
Dosage and administration
inside
Ulcavis® adults are prescribed 1 tablet 4 times a day 30 minutes before meals and at night, or 2 tablets 2 times a day 30 minutes before meals.
Tablets should be taken 30 minutes before meals with a small amount of water.
The duration of the course of treatment is 4-8 weeks.
For the next 8 weeks, preparations containing bismuth should not be used.
For the eradication of Helicobacter pylori, it is advisable to use Ulcavis® in combination with other antibacterial agents with anti-Helicobacter pylori activity and proton pump inhibitors, the so-called quadruple therapy.
Possible side effects
Very common (≥ 1/10)
-staining of feces in a dark color due to the formation of bismuth sulfide, but it can be easily distinguished from melena
Uncommon (≥ 1/1000, <1/100)
- nausea, vomiting, diarrhea or constipation
- allergic reactions: skin rash, itching
Very rare (<1/10000)
- anaphylactic reactions
- with prolonged use in high doses - encephalopathy associated with the accumulation of bismuth in the central nervous system
Contraindications
- Hypersensitivity to any component of the drug
- Chronic renal failure
- hypokalemia
- Pregnancy and lactation
- Children's age up to 18 years
Drug Interactions
Within half an hour after taking Ulcavis®, it is not recommended to use other medicines orally, as well as the intake of food and liquids, in particular, antacids, milk, fruits and fruit juices. This is due to the fact that they, when taken orally, can affect the effectiveness of Ulcavis®.
Ulcavis® reduces the absorption of tetracycline.
Special instructions
The drug should not be used for more than 8 weeks, it is also not recommended to exceed the established daily doses for adults during treatment. During the period of drug treatment, other drugs containing bismuth should not be used, as the risk of side effects increases. At the end of the course treatment with the drug in recommended doses, the concentration of the active active substance in the blood plasma does not exceed 3-58 μg / l, and intoxication is observed only at a concentration above 100 mg / l.
Prolonged use of large doses of bismuth-containing compounds is not recommended, since in some cases this can lead to reversible encephalopathy. The risk of encephalopathy, however, is minimal when Ulcavis is used at the recommended doses.
When using Ulcavis, staining of feces in a dark color is possible due to the formation of bismuth sulfide. Sometimes there is a darkening of the tongue. It is not recommended to drink alcohol during therapy with Ulcavis.
Features of the effect of the drug on the ability to drive a vehicle or potentially dangerous mechanisms
Does not affect.
Overdose
Symptoms: dyspepsia, rash, inflammation of the mucous membranes of the mouth, characteristic darkening in the form of blue lines on the gums, impaired renal function.
Treatment: drug withdrawal, gastric lavage, activated charcoal, saline laxatives. The control of kidney function, the concentration of bismuth in the blood and urine is shown. In the future, symptomatic therapy is carried out. In case of impaired renal function, accompanied by a high level of bismuth in the blood plasma, it is possible to introduce complexing agents - dimercaptosuccinic and dimercaptopropanesulfonic acids. In severe renal failure, hemodialysis is indicated.
Storage conditions
Store in original packaging at a temperature not exceeding 25 ºС.
Keep out of the reach of children!
Shelf life - 2 years
Do not use after the expiration date