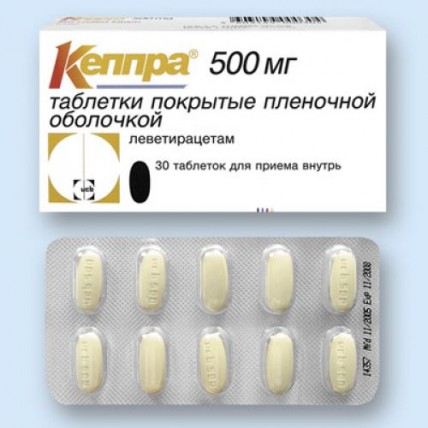
Keppra® 30s 500 mg film-coated tablets
- $63.60
The instruction for medical use
of Keppra® medicine
the Trade name
of Keppra®
the International unlicensed
name Levetiratsetam Lekarstvennaya
the Tablet form, film coated, 250 mg, 500 mg, 1000 mg
Structure
One tablet of 250 mg contains
active agent – to levetiratseta of 250 mg,
excipients: sodium of a kroskarmelloz, macrogoal 6000, silicon dioxide colloidal anhydrous, magnesium stearate,
structure of a cover of Opadray 85F20694 blue: FD&C dye blue No. 2/indigo carmine aluminum varnish (E132), makrogol / polyethyleneglycol 3350, the polyvinyl alcohol which is partially hydrolyzed, talc, the titan dioxide (E171).
One tablet of 500 mg contains
active agent - to levetiratseta of 500 mg,
excipients: sodium of a kroskarmelloz, macrogoal 6000, silicon dioxide colloidal anhydrous, magnesium stearate,
structure of a cover of Opadray 85F32004 yellow: ferrous oxide yellow (E172), makrogol / polyethyleneglycol 3350, the polyvinyl alcohol which is partially hydrolyzed, talc, the titan dioxide (E171).
One tablet of 1000 mg contains
active agent – to levetiratseta of 1000 mg,
excipients: sodium of a kroskarmelloz, macrogoal 6000, silicon dioxide colloidal anhydrous, magnesium stearate,
structure of a cover of Opadray 85 F 18422 white: makrogol / polyethyleneglycol 3350, the polyvinyl alcohol which is partially hydrolyzed, talc, the titan dioxide (E171).
The description
of the Tablet of 250 mg
of the Tablet of oblong shape, film coated blue color, with risky for a break, the squeezed-out inscription ucb and figure 250 on one party of a tablet
of the Tablet of 500 mg
of the Tablet of oblong shape, film coated yellow color, with risky for a break, the squeezed-out inscription ucb and figure 500 on one party of a tablet
of the Tablet of 1000 mg
of the Tablet of oblong shape, film coated white color, with risky for a break, the squeezed-out inscription ucb and figure 1000 on one party of a tablet
Pharmacotherapeutic group
Antiepileptic drugs. Antiepileptic drugs others. Levetiratsetam.
ATH N03AX14 code
the Pharmacological
Pharmacokinetics Absorption Later properties of intake to levetiratseta is quickly soaked up from digestive tract. Absorption full also has linear character that allows to predict concentration of drug in serum on the basis of the accepted dose of a levetiratsetam expressed in body weight mg/kg. Extent of absorption does not depend on a dose and meal. The bioavailability is about 100%. The maximum concentration in serum (Cmax) is reached in 1.3 hours after oral administration of a dose of 1000 mg and makes 31 mkg/ml, after a repeated dose – 43 mkg/ml. The equilibrium state is reached in 2 days, concentration makes 270 ng/ml, after repeated use of a dose of 1000 mg – 308 ng/ml. Constant concentration are reached in 2 days at use twice a day.
The pharmacokinetics of a levetiratsetam at children has linear character in the range of doses of 20-60 mg/kg/day, Cmax is reached in 0.5-1 h.
Distribution
Extent of binding of a levetiratsetam and its main metabolite with proteins of blood plasma less than 10%. The volume of distribution (Vd) is about 0.5-0.7 l/kg that to similarly total amount of liquid of an organism.
Metabolism
Levetiratsetam is not metabolized extensively in a human body. The main mechanism of metabolism (24%) is fermental hydrolysis of atsetamidny group which metabolites are found in the majority of fabrics, including blood cells. Formation of the basic, pharmacological an inactive metabolite (ucb L057), happens without liver P450 cytochrome participation.
In vitro to levetiratseta and its primary metabolites are not suppressed with isoforms of P450 cytochrome (CYP3A4, 2A6, 2C9, 2C19, 2D6, 2E1, 1A2), glyukuronidtransferazam (UGT1A1, UGT1A6) and epoxide with a hydroxylase. Levetiratsetam does not influence a valproic acid glyukuronidation.
In the cellular culture of hepatocytes to levetiratseta has no or has very low extent of influence on CYP2B6 and CYP3A4. Considerable interaction between levetiratsetamy and oral contraceptives, warfarin and digoxin is not expected. Thus, interaction of a levetiratsetam with other substances is improbable.
Removal
makes Elimination half-life (T½) at adults 7 ± 1 h and does not depend on a method of administration and the mode of use. The average system clearance is 0.96 ml/min. 95% of drug are removed by kidneys. Discharge with excrements makes about 0.3% of the accepted dose. Cumulative removal of a levetiratsetam and its primary metabolites with urine makes 24-66% of the accepted dose, mainly within the first 48 hours. The renal clearance of a levetiratsetam and its metabolite is equal to 0.6 and 4.2 ml/min., respectively.
Changes of clearance after repeated reception are not observed.
A floor,
Paule's race and race do not influence pharmacokinetics of a levetiratsetam.
Elderly patients
At elderly patients of T½ increases by 40% (up to 10–11 h) that is connected with deterioration in renal functions at patients of this group.
The renal failure
At patients with disturbances of functions of kidneys clearance of a levetiratsetam and its main metabolite correlates with clearance of creatinine therefore patients with a renal failure are recommended to select a dose according to clearance of creatinine. At adults with a renal failure on an end-stage of T½ makes 25 hours during the period between sessions of dialysis and 3.1 hours - during dialysis. During the 4-hour session of dialysis about 51% of a levetiratsetam are removed.
The abnormal liver function
At patients with insignificant or moderate abnormal liver functions of significant changes in clearance of a levetiratsetam is not observed. In most cases in heavy abnormal liver functions and the accompanying renal failure the clearance of a levetiratsetam decreases by 50%, generally because of the accompanying deterioration in renal clearance.
Children
of T½ at children (from 6 to 12 years) after oral administration of a single dose of 20 mg/kg makes about 5-6 hours. The system clearance at children is about 30% higher, than at adults and directly depends on body weight. After repeated intake of drug (from 20 to 60 mg/kg/days) by children with epilepsy (from 6 to 12 years), to levetiratseta it is quickly soaked up. The peak of concentration in plasma is observed from 0.5 to 1.0 hours after administration of drug. Linear and dozo-proportional increase in peak of concentration in plasma and the area under a curve was observed. Elimination half-life makes about 5 hours. The obvious clearance was 1.1 ml/min.
The pharmacodynamics
Levetiratsetam, active ingredient of the drug Keppra®, represents derivative a pirrolidon (S-enantiomer of a α-etil-2-okso-1-pirrolidinatsetamid) different from the known antiepileptic drugs on structure. The mechanism of action of a levetiratsetam is studied not completely, but obviously differs from mechanisms of action of the existing antiepileptic means.
Data of the researches in vitro and in Vivo allow to assume what to levetiratseta does not affect the main cellular characteristics and normal neurotransmission.
In the researches in vitro it was shown what to levetiratseta influences concentration of ions of Ca2 + in neurons, partially interfering with current of ions of Ca2 + via channels of N-type and suppressing release of calcium from vnutrineyronalny depots. Besides, to levetiratseta partially restores current through GAMK- and glycine - dependent channels, suppressed by effect of zinc and β-Carbolenums.
One of expected mechanisms of action is based on the proved binding of connection with a glycoprotein of synoptic vesicles of SV2A which are in gray matter of a head and spinal cord. Presumably the anti-convulsive action which is expressed in prevention of hyper synchronization of neural activity is explained by it. Besides, to levetiratseta influences GAMK- and glycine receptors, modulating them by means of various endogenous connections. Levetiratsetam does not influence normal neurotransmission, but suppresses the epileptiform neuronalny flashes induced by GAMK agonist bikukulliny and also excitement of glutamate receptors. The activity of drug is proved as concerning focal, and generalized epileptic attacks (epileptiform manifestations / photoparoxysmal reaction).
Indications
as monotherapy at treatment:
- or without it at adults and teenagers of 16 years is also more senior than partial attacks with secondary generalization with for the first time the diagnosed epilepsy
as auxiliary therapy at treatment:
- with secondary generalization or without it at adults, teenagers and children 1 years with epilepsy are more senior than partial attacks
- at adults and children 12 years with juvenile myoclonic epilepsy are more senior than myoclonic spasms
- at adults and children 12 years with idiopathic generalized epilepsy
At patients with the complicated act of swallowing perhaps use of drug in the form of injections are more senior than primary and generalized toniko-clonic attacks (adults and children are more senior than 4 years).
The route of administration and doses
Therapy can begin with intravenous or oral administration. Transition to other form of introduction has to be carried out directly, without titration of a dose. It is necessary to adhere to the general daily dose and frequency of introduction. A pill of the drug Keppra® is taken orally, irrespective of meal. The daily dose is divided into two identical receptions. Tablets are washed down with enough liquid.
Monotherapy
Treatment of adults and teenagers is more senior than 16 years begin with the daily dose 500 mg divided into two receptions (on ½ tablets of 500 mg 2 times a day). In 2 weeks the dose can be increased to an initial therapeutic dose of 1000 mg (on 500 mg twice a day). Further the dose can increase by 250 mg each 2 weeks, depending on the clinical response to therapy. The maximum daily dose makes 3000 mg (on 1500 mg twice a day).
As auxiliary therapy
the Adults and teenagers of 18 years are also more senior also teenagers from 12 to 17 years with the body weight of 50 kg or
it is necessary to begin more Treatment with the daily dose 1000 mg divided into two receptions (on 500 mg twice a day). This dose has to be appointed in the first day of a course.
Depending on the clinical answer and tolerance of drug, the dose can be increased up to 1500 mg appointed twice a day. Twice a day it is possible to carry out dose adjustment (reduction or increase) with a step of 500 mg each 2-4 weeks.
Children
the Doctor has to appoint drug in the most suitable dosage form and a dosage, proceeding from the body weight of the patient, age and a necessary therapeutic dose.
The tableted form of drug is not intended for use at children 6 years are younger. Also tableted form of drug is not suitable for children with body weight less than 25 kg, to the patients experiencing difficulties with the act of swallowing, or that to whom purpose of a dose of drug less than 250 mg is shown. In all listed above cases it is necessary to use drug in the form of solution for intake.
Monotherapy
Efficiency and safety of Keppry® at children and teenagers is younger than 16 years as monotherapy is not established.
Auxiliary therapy for children and teenagers with body weight less than 50 kg
the Initial dose makes 10 mg/kg of body weight twice a day. Depending on the clinical answer and tolerance of drug it is possible to increase a dose to 30 mg/kg of body weight twice a day. The dose step for correction should not exceed 10 mg/kg of body weight twice in every day 2 weeks. It is necessary to apply a minimal effective dose.
The drug dose at children with the body weight of 50 kg and is similar to adults above.
Recommendations about a dosage for children and teenagers
Weight
Starting dose: Two times pass 10 mg/kg
the Maximum dose: 30 mg/kg twice a day
25
kg (1) 250 mg twice a day
750 mg twice a day
from 50
kg (2) 500 mg twice a day
1500 mg twice a day
(1) - to children up to 25 kg or less, it is necessary to begin treatment levetiratsetamy with solution for intake of 100
mg/ml (2) - a dosage for children and teenagers weighing from 50 kg or more same, as at adults.
Elderly patients
Correction of a dose is recommended in case of a renal failure.
Patients with a renal failure
the Daily dose has to be individualized according to degree of renal dysfunction.
As to levetiratseta it is removed by kidneys, patients with a renal failure need to select a dose, proceeding from clearance of creatinine.
The Clearance of Creatinine (CC) for adults and teenagers with body weight more than 50 kg can be calculated on the basis of concentration of creatinine in serum by the following formula:
× the body weight (kg)
of KK (ml/min.) =------------------------------------------------------
72 × serumal KK (mg/dl)
Clearance of Creatinine at women is calculated by multiplication of the received value by 0.85.
Correction on the body surface area (BSA) is made on the following formula:
KK (ml/min.)
of KK (ml/min. / 1.73 m ²) =--------------------------x 1.73
PPTpatsienta (m ²)
the Scheme of dosing for adults and teenagers weighing 50 kg and more with renal failures
the Renal failure
of KK (ml/min.)
(ml/min. / 1.73 m ²)
the Scheme of dosing
Normal function of kidneys
& gt,
80 500-1500 mg twice a day
Insignificant extent of disturbance
of 50-79 500-1000 mg twice a day
Moderate extent of disturbance
of 30-49 250-750 mg twice a day
Heavy extent of disturbance
& lt,
30,250-500 mg twice a day
the End-stage – patients on dialysis *
-
500–1000 mg **
* In the first day of treatment is recommended once a day reception of the saturating dose of 750 mg
** After dialysis the reception of an additional dose of 250-500 mg
the Liver failure is recommended
In weak and moderate abnormal liver functions the dose adjustment is not required. At patients with heavy abnormal liver functions the decrease in clearance of creatinine can display not fully extent of disturbance of functions of kidneys therefore to patients with clearance of creatinine & lt, / 1.73 sq.m a daily dose it is recommended to lower 60 ml/min. by 50%.
At children with renal failures the dose of a levetiratsetam needs to be adjusted according to degree of a renal failure.
The Clearance of Creatinine (CC) in ml/min. / 1.73 the sq.m at teenagers, children and babies can be calculated on the basis of the serumal level of creatinine (mg/dl) on the following formula (Schwartz's formula):
Growth (cm) x
ks KK (ml/min. / 1.73 m ²) =-------------------------
Creatinine of serum (mg/dl)
Ks=0.55 in case of children up to 13 years and teenage girls, in case of teenage boys of ks=0.7.
The scheme of dosing at children and teenagers with body weight is less than 50 kg with a renal failure
the Stage
Clearance of Creatinine (ml/min. / 1.73 of sq.m)
the Dose and frequency of use
Children and teenagers with
body weight less than 50 kg
Normal function of kidneys
& gt, 80
10–30 mg/kg twice a day
Insignificant extent of disturbance
of 50-79
10–20 mg/kg twice a day
Moderate extent of disturbance
of 30-49
5–15 mg/kg twice a day
Heavy extent of disturbance
& lt, 30
5–10 mg/kg twice a day
the Patients with an end-stage of a renal failure who are on dialysis
-
10–20 mg/kg
(1) (2) (1) V the first day of use of a levetiratsetam are recommended once a day reception of a load dose of 15 mg/kg.
(2) After dialysis the reception of an additional dose of 5-10 mg/kg is recommended.
Side effects
the Profile of side reactions is based on the analysis of these placebos - controlled clinical trials with coverage of 3416 patients. The nasopharyngitis, fatigue, a headache, drowsiness, dizziness were the most frequent side reactions.
The undesirable reactions given below are listed according to defeat of bodies and the systems of bodies and occurrence frequency. Frequency of occurrence is defined as follows: very often (≥ 1/10), it is frequent (≥ 1/100 and & lt, 1/10), infrequently (≥ 1/1,000 and & lt, 1/100), is rare (≥ 1/10,000 and & lt, 1/1,000), is very rare (& lt, 1/10,000).
Very often
- a nasopharyngitis
- drowsiness
- a headache
Often
- anorexia (is more often at the accompanying appointment with topiramaty)
- a depression, aggression, concern, insomnia, nervousness, irritability, emotional lability / change of mood,
- spasms, balance disturbance, dizziness, a lethargy, a tremor
- vertigo
- cough
- pain in a stomach, diarrhea, dyspepsia, nausea, vomiting
- rash
- asthenia/fatigue
Infrequently
- a leukopenia, thrombocytopenia
- decrease/increase in body weight
- attempt of a suicide, suicide thoughts, mental disorders, a behavior disorder, hallucinations, anger, confusion of consciousness, the panic attacks, emotional lability / change of mood, excitement
- amnesia, disturbance of memory, an ataxy/incoordination, paresthesia, attention disorder
- a diplopia, illegibility of sight
- pathological results of hepatic tests
- an alopecia (regression at drug withdrawal), eczema, an itching
- muscle weakness, myalgia
- injuries
Seldom
- infections
- a pancytopenia (in certain cases was followed by marrow myelosuppression), a neutropenia, an agranulocytosis
- medicinal reactions in the form of an eosinophilia and system reactions (DRESS)
- a hyponatremia
- a suicide, pathological thinking, disorder of the personality,
- a choreoathetosis, dyskinesia, a hyperkinesia
- pancreatitis
- a liver failure, hepatitis
- a toxic epidermal necrolysis, Stephens-Johnson's syndrome, a multiformny erythema
When comparing of the data obtained at treatment of 645 children aged from 4 up to 16 years it was revealed that the profile of side reactions between children and adults is similar except for behavioural and mental disorders which meet bigger frequency at children. The following side reactions meet at this cohort more often than at adults: vomiting, alarm, changes of mood, lability, aggression, atypical behavior, lethargy.
Contraindications
- hypersensitivity to a levetiratsetam, other derivatives of a pirrolidon or any auxiliary component of drug
- the children's age up to 6 years (use of drug in the form of solution for intake)
- children's and teenage age up to 18 years (for tablets with a dose of 250 mg is recommended)
Medicinal interactions
the Antiepileptic
drugs Levetiratsetam does not affect concentration of the known antiepileptic drugs in blood serum (Phenytoinum, carbamazepine, valproic acid, phenobarbital, lamotrigin, topiramat, gabapentin and Primidonum).
As well as at adults, in children's population the considerable interaction of medicines when prescribing the drug Keppra® in a dose of 60 mg/kg/days was not observed.
Assessment of indicators of pharmacokinetics at children and teenagers with epilepsy (4-17 years) confirmed that additional therapy by the drug Keppra® at intake did not affect concentration of the drugs of carbamazepine and Valproatum accepted together with levetiratsetamy. Nevertheless, the clearance of a levetiratsetam at the children receiving enzyme - the induced antiepileptic means, was 20% higher, than at children who did not take such drugs. Dose adjustment is not required.
Probenetsid
Probenetsid (blocker of renal canalicular secretion) at use on 500 mg four times a day inhibits renal clearance of primary metabolite, but not the levetiratsetam. Nevertheless, concentration of this metabolite remains low. It is expected that other medicines excreted by active tubular secretion also can reduce renal clearance of a metabolite. Influence of a levetiratsetam on pharmacokinetics of a probenetsid and other actively cosecreted drugs, such as non-steroidal anti-inflammatory drugs, sulfonamides and methotrexate is unknown.
Oral contraceptives, digoxin and warfarin
the Drug Keppra® in a daily dose of 1000 mg does not influence pharmacokinetics of oral contraceptives (ethinylestradiol and levonorgestrel).
The drug Keppra® in a daily dose of 2000 mg does not influence pharmacokinetics of digoxin and warfarin.
Digoxin, oral contraceptives and warfarin do not influence pharmacokinetics of a levetiratsetam.
Antacids
Are not present data on influence of antacids on process of absorption of a levetiratsetam.
Laxatives
At a concomitant use with an osmotic laxative macrogoal – the efficiency of a levetiratsetam decreases in this connection the macrogoal should be accepted in 1 hour prior to or 1 hour after administration of drug of Keppra®.
Food and alcohol
Meal does not influence extent of absorption of a levetiratsetam, however absorption speed slows down a little. Data on interaction of the drug Keppra® with alcohol are absent.
The special
instructions Drug Withdrawal According to the current clinical practice, in case of need drug withdrawals it it is necessary to make gradually (lowering a single dose by 500 mg each 2-4 weeks at patients with body weight more than 50 kg). At children and teenagers with body weight less than 50 kg the dose decline should not exceed 10 mg/kg of body weight twice a day each 2 weeks.
The renal or liver failure
Prescribing of the drug Keppra® to patients with a renal or liver failure can demand drug dose adjustment. At patients with heavy degree of a liver failure it is necessary to estimate functions of kidneys before therapy.
A depression / suicide thoughts
Proceeding from the received messages about suicide cases, suicide intentions and attempts of a suicide against the background of use of antiepileptic drugs, including levetiratseta, the patients and persons who are looking after them should be warned about need to report to the attending physician about emergence of any symptoms of a depression or suicide intentions. Patients have to be under medical observation regarding emergence of suicide intentions and attempts of a suicide though assessment of risk of increase in these disorders against the background of intake of antiepileptic drugs showed that degree of risk increases slightly. The origins of these risks are unknown.
Children
the Tableted form of the drug Keppra® is not recommended to use for children 6 years are younger.
The existing data on use of drug for children do not indicate any undesirable impact on development and maturing. However, the remote effects of use of drug on ability to training, intellectual development, growth, function of closed glands, puberty and fertility remain unknown.
The efficiency and safety of a levetiratsetam at children are younger than 1 year as auxiliary therapy at partial attacks with secondary generalization or without it is not established.
The efficiency and safety of a levetiratsetam as auxiliary therapy at children are younger than 12 years with juvenile myoclonic epilepsy ilis idiopathic generalized epilepsy is not established.
Pregnancy and the period of a lactation
Fertility
of the Research on animals showed lack of influence on fertility. Clinical data are absent, the potential risk for the person is unknown.
Pregnancy
In post-marketing prospective registers cases of use of drug for pregnant women in the first trimester are described. In spite of the fact that by results of these data in general increase in frequency of congenital anomalies was not traced, the teratogenic risk cannot be excluded. At co-administration of several antiepileptic drugs the risk of congenital anomalies, in communication increases with what monotherapy has to be considered.
Researches on animals showed reproductive toxicity.
It is not recommended to use the drug Keppra® at the pregnant women and women of childbearing age who are not applying contraception methods except for cases of clinical need.
As well as with other antiepileptic drugs, physiological changes during pregnancy can affect concentration of a levetiratsetam (decrease in level in blood serum). The most significant decrease is observed in the third trimester (up to 60% of initial concentration).
The women receiving to levetiratseta during pregnancy have to be provided with the corresponding clinical inspection. Breaks in treatment by antiepileptic means can lead to exacerbation of a disease that can be injurious to health of mother and a fruit.
The lactation period
Levetiratsetam is excreted in breast milk. Feeding by a breast during treatment is not recommended. However, if drug Keppra® use by nursing mothers is necessary, it is necessary to carry out the assessment of advantage and risk of reception/drug withdrawal.
The drug Keppra® is not recommended during the lactation period if there is no clinical need.
Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
Influence of the drug Keppra® on ability to drive the car or to work with mechanisms specially was not studied. However, in connection with different individual sensitivity to drug from the central nervous system, especially at the beginning of therapy or at increase in doses, it is necessary to refrain from control of motor transport and perhaps dangerous works demanding special attentiveness and speed of psychomotor reaction.
Overdose
Symptoms: drowsiness, excitement, consciousness oppression, respiratory depression, coma.
Treatment: in an acute period – an artificial call of vomiting and gastric lavage with the subsequent use of activated carbon. There is no specific antidote for a levetiratsetam. If necessary provide symptomatic treatment in clinic using a hemodialysis (efficiency of dialysis for a levetiratsetam – 60%, for its main metabolite – 74%).
Form of release and packing
of the Tablet, film coated, 250 mg, 500 mg, 1000 mg.
On 10 tablets place in blister strip packaging from a film of polyvinylchloride and aluminum foil. On 3 or 6 blister strip packagings together with the instruction for medical use in the state and Russian languages place in a cardboard pack.
To Store storage conditions in the dry place at a temperature not above 25 °C.
To store out of children's reach!
A period of storage
3 years
not to use after the termination of an expiration date
Prescription status
According to the prescription
YuSB Producer Pharma S.A., Belgium
of Allee da la Recherche 60, Brussels
the Owner of the registration certificate
GlaksoSmitKlyayn Export of Ltd., Great Britain
980 Great West Road, Brentford, Middlesex, TW8 9GS,
the UK Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods)
of Predstavitelstvo GlaksoSmitKlyayn Export Ltd in
Kazakhstan 050059, Almaty, Furmanov St., 273
Phone number: +7 727 258 28 92, +7 727 259 09 96
Fax number: + 7 727 258 28 90
E-mail address:
To Develop kaz.med@gsk.com
of Keppra® medicine
the Trade name
of Keppra®
the International unlicensed
name Levetiratsetam Lekarstvennaya
the Tablet form, film coated, 250 mg, 500 mg, 1000 mg
Structure
One tablet of 250 mg contains
active agent – to levetiratseta of 250 mg,
excipients: sodium of a kroskarmelloz, macrogoal 6000, silicon dioxide colloidal anhydrous, magnesium stearate,
structure of a cover of Opadray 85F20694 blue: FD&C dye blue No. 2/indigo carmine aluminum varnish (E132), makrogol / polyethyleneglycol 3350, the polyvinyl alcohol which is partially hydrolyzed, talc, the titan dioxide (E171).
One tablet of 500 mg contains
active agent - to levetiratseta of 500 mg,
excipients: sodium of a kroskarmelloz, macrogoal 6000, silicon dioxide colloidal anhydrous, magnesium stearate,
structure of a cover of Opadray 85F32004 yellow: ferrous oxide yellow (E172), makrogol / polyethyleneglycol 3350, the polyvinyl alcohol which is partially hydrolyzed, talc, the titan dioxide (E171).
One tablet of 1000 mg contains
active agent – to levetiratseta of 1000 mg,
excipients: sodium of a kroskarmelloz, macrogoal 6000, silicon dioxide colloidal anhydrous, magnesium stearate,
structure of a cover of Opadray 85 F 18422 white: makrogol / polyethyleneglycol 3350, the polyvinyl alcohol which is partially hydrolyzed, talc, the titan dioxide (E171).
The description
of the Tablet of 250 mg
of the Tablet of oblong shape, film coated blue color, with risky for a break, the squeezed-out inscription ucb and figure 250 on one party of a tablet
of the Tablet of 500 mg
of the Tablet of oblong shape, film coated yellow color, with risky for a break, the squeezed-out inscription ucb and figure 500 on one party of a tablet
of the Tablet of 1000 mg
of the Tablet of oblong shape, film coated white color, with risky for a break, the squeezed-out inscription ucb and figure 1000 on one party of a tablet
Pharmacotherapeutic group
Antiepileptic drugs. Antiepileptic drugs others. Levetiratsetam.
ATH N03AX14 code
the Pharmacological
Pharmacokinetics Absorption Later properties of intake to levetiratseta is quickly soaked up from digestive tract. Absorption full also has linear character that allows to predict concentration of drug in serum on the basis of the accepted dose of a levetiratsetam expressed in body weight mg/kg. Extent of absorption does not depend on a dose and meal. The bioavailability is about 100%. The maximum concentration in serum (Cmax) is reached in 1.3 hours after oral administration of a dose of 1000 mg and makes 31 mkg/ml, after a repeated dose – 43 mkg/ml. The equilibrium state is reached in 2 days, concentration makes 270 ng/ml, after repeated use of a dose of 1000 mg – 308 ng/ml. Constant concentration are reached in 2 days at use twice a day.
The pharmacokinetics of a levetiratsetam at children has linear character in the range of doses of 20-60 mg/kg/day, Cmax is reached in 0.5-1 h.
Distribution
Extent of binding of a levetiratsetam and its main metabolite with proteins of blood plasma less than 10%. The volume of distribution (Vd) is about 0.5-0.7 l/kg that to similarly total amount of liquid of an organism.
Metabolism
Levetiratsetam is not metabolized extensively in a human body. The main mechanism of metabolism (24%) is fermental hydrolysis of atsetamidny group which metabolites are found in the majority of fabrics, including blood cells. Formation of the basic, pharmacological an inactive metabolite (ucb L057), happens without liver P450 cytochrome participation.
In vitro to levetiratseta and its primary metabolites are not suppressed with isoforms of P450 cytochrome (CYP3A4, 2A6, 2C9, 2C19, 2D6, 2E1, 1A2), glyukuronidtransferazam (UGT1A1, UGT1A6) and epoxide with a hydroxylase. Levetiratsetam does not influence a valproic acid glyukuronidation.
In the cellular culture of hepatocytes to levetiratseta has no or has very low extent of influence on CYP2B6 and CYP3A4. Considerable interaction between levetiratsetamy and oral contraceptives, warfarin and digoxin is not expected. Thus, interaction of a levetiratsetam with other substances is improbable.
Removal
makes Elimination half-life (T½) at adults 7 ± 1 h and does not depend on a method of administration and the mode of use. The average system clearance is 0.96 ml/min. 95% of drug are removed by kidneys. Discharge with excrements makes about 0.3% of the accepted dose. Cumulative removal of a levetiratsetam and its primary metabolites with urine makes 24-66% of the accepted dose, mainly within the first 48 hours. The renal clearance of a levetiratsetam and its metabolite is equal to 0.6 and 4.2 ml/min., respectively.
Changes of clearance after repeated reception are not observed.
A floor,
Paule's race and race do not influence pharmacokinetics of a levetiratsetam.
Elderly patients
At elderly patients of T½ increases by 40% (up to 10–11 h) that is connected with deterioration in renal functions at patients of this group.
The renal failure
At patients with disturbances of functions of kidneys clearance of a levetiratsetam and its main metabolite correlates with clearance of creatinine therefore patients with a renal failure are recommended to select a dose according to clearance of creatinine. At adults with a renal failure on an end-stage of T½ makes 25 hours during the period between sessions of dialysis and 3.1 hours - during dialysis. During the 4-hour session of dialysis about 51% of a levetiratsetam are removed.
The abnormal liver function
At patients with insignificant or moderate abnormal liver functions of significant changes in clearance of a levetiratsetam is not observed. In most cases in heavy abnormal liver functions and the accompanying renal failure the clearance of a levetiratsetam decreases by 50%, generally because of the accompanying deterioration in renal clearance.
Children
of T½ at children (from 6 to 12 years) after oral administration of a single dose of 20 mg/kg makes about 5-6 hours. The system clearance at children is about 30% higher, than at adults and directly depends on body weight. After repeated intake of drug (from 20 to 60 mg/kg/days) by children with epilepsy (from 6 to 12 years), to levetiratseta it is quickly soaked up. The peak of concentration in plasma is observed from 0.5 to 1.0 hours after administration of drug. Linear and dozo-proportional increase in peak of concentration in plasma and the area under a curve was observed. Elimination half-life makes about 5 hours. The obvious clearance was 1.1 ml/min.
The pharmacodynamics
Levetiratsetam, active ingredient of the drug Keppra®, represents derivative a pirrolidon (S-enantiomer of a α-etil-2-okso-1-pirrolidinatsetamid) different from the known antiepileptic drugs on structure. The mechanism of action of a levetiratsetam is studied not completely, but obviously differs from mechanisms of action of the existing antiepileptic means.
Data of the researches in vitro and in Vivo allow to assume what to levetiratseta does not affect the main cellular characteristics and normal neurotransmission.
In the researches in vitro it was shown what to levetiratseta influences concentration of ions of Ca2 + in neurons, partially interfering with current of ions of Ca2 + via channels of N-type and suppressing release of calcium from vnutrineyronalny depots. Besides, to levetiratseta partially restores current through GAMK- and glycine - dependent channels, suppressed by effect of zinc and β-Carbolenums.
One of expected mechanisms of action is based on the proved binding of connection with a glycoprotein of synoptic vesicles of SV2A which are in gray matter of a head and spinal cord. Presumably the anti-convulsive action which is expressed in prevention of hyper synchronization of neural activity is explained by it. Besides, to levetiratseta influences GAMK- and glycine receptors, modulating them by means of various endogenous connections. Levetiratsetam does not influence normal neurotransmission, but suppresses the epileptiform neuronalny flashes induced by GAMK agonist bikukulliny and also excitement of glutamate receptors. The activity of drug is proved as concerning focal, and generalized epileptic attacks (epileptiform manifestations / photoparoxysmal reaction).
Indications
as monotherapy at treatment:
- or without it at adults and teenagers of 16 years is also more senior than partial attacks with secondary generalization with for the first time the diagnosed epilepsy
as auxiliary therapy at treatment:
- with secondary generalization or without it at adults, teenagers and children 1 years with epilepsy are more senior than partial attacks
- at adults and children 12 years with juvenile myoclonic epilepsy are more senior than myoclonic spasms
- at adults and children 12 years with idiopathic generalized epilepsy
At patients with the complicated act of swallowing perhaps use of drug in the form of injections are more senior than primary and generalized toniko-clonic attacks (adults and children are more senior than 4 years).
The route of administration and doses
Therapy can begin with intravenous or oral administration. Transition to other form of introduction has to be carried out directly, without titration of a dose. It is necessary to adhere to the general daily dose and frequency of introduction. A pill of the drug Keppra® is taken orally, irrespective of meal. The daily dose is divided into two identical receptions. Tablets are washed down with enough liquid.
Monotherapy
Treatment of adults and teenagers is more senior than 16 years begin with the daily dose 500 mg divided into two receptions (on ½ tablets of 500 mg 2 times a day). In 2 weeks the dose can be increased to an initial therapeutic dose of 1000 mg (on 500 mg twice a day). Further the dose can increase by 250 mg each 2 weeks, depending on the clinical response to therapy. The maximum daily dose makes 3000 mg (on 1500 mg twice a day).
As auxiliary therapy
the Adults and teenagers of 18 years are also more senior also teenagers from 12 to 17 years with the body weight of 50 kg or
it is necessary to begin more Treatment with the daily dose 1000 mg divided into two receptions (on 500 mg twice a day). This dose has to be appointed in the first day of a course.
Depending on the clinical answer and tolerance of drug, the dose can be increased up to 1500 mg appointed twice a day. Twice a day it is possible to carry out dose adjustment (reduction or increase) with a step of 500 mg each 2-4 weeks.
Children
the Doctor has to appoint drug in the most suitable dosage form and a dosage, proceeding from the body weight of the patient, age and a necessary therapeutic dose.
The tableted form of drug is not intended for use at children 6 years are younger. Also tableted form of drug is not suitable for children with body weight less than 25 kg, to the patients experiencing difficulties with the act of swallowing, or that to whom purpose of a dose of drug less than 250 mg is shown. In all listed above cases it is necessary to use drug in the form of solution for intake.
Monotherapy
Efficiency and safety of Keppry® at children and teenagers is younger than 16 years as monotherapy is not established.
Auxiliary therapy for children and teenagers with body weight less than 50 kg
the Initial dose makes 10 mg/kg of body weight twice a day. Depending on the clinical answer and tolerance of drug it is possible to increase a dose to 30 mg/kg of body weight twice a day. The dose step for correction should not exceed 10 mg/kg of body weight twice in every day 2 weeks. It is necessary to apply a minimal effective dose.
The drug dose at children with the body weight of 50 kg and is similar to adults above.
Recommendations about a dosage for children and teenagers
Weight
Starting dose: Two times pass 10 mg/kg
the Maximum dose: 30 mg/kg twice a day
25
kg (1) 250 mg twice a day
750 mg twice a day
from 50
kg (2) 500 mg twice a day
1500 mg twice a day
(1) - to children up to 25 kg or less, it is necessary to begin treatment levetiratsetamy with solution for intake of 100
mg/ml (2) - a dosage for children and teenagers weighing from 50 kg or more same, as at adults.
Elderly patients
Correction of a dose is recommended in case of a renal failure.
Patients with a renal failure
the Daily dose has to be individualized according to degree of renal dysfunction.
As to levetiratseta it is removed by kidneys, patients with a renal failure need to select a dose, proceeding from clearance of creatinine.
The Clearance of Creatinine (CC) for adults and teenagers with body weight more than 50 kg can be calculated on the basis of concentration of creatinine in serum by the following formula:
× the body weight (kg)
of KK (ml/min.) =------------------------------------------------------
72 × serumal KK (mg/dl)
Clearance of Creatinine at women is calculated by multiplication of the received value by 0.85.
Correction on the body surface area (BSA) is made on the following formula:
KK (ml/min.)
of KK (ml/min. / 1.73 m ²) =--------------------------x 1.73
PPTpatsienta (m ²)
the Scheme of dosing for adults and teenagers weighing 50 kg and more with renal failures
the Renal failure
of KK (ml/min.)
(ml/min. / 1.73 m ²)
the Scheme of dosing
Normal function of kidneys
& gt,
80 500-1500 mg twice a day
Insignificant extent of disturbance
of 50-79 500-1000 mg twice a day
Moderate extent of disturbance
of 30-49 250-750 mg twice a day
Heavy extent of disturbance
& lt,
30,250-500 mg twice a day
the End-stage – patients on dialysis *
-
500–1000 mg **
* In the first day of treatment is recommended once a day reception of the saturating dose of 750 mg
** After dialysis the reception of an additional dose of 250-500 mg
the Liver failure is recommended
In weak and moderate abnormal liver functions the dose adjustment is not required. At patients with heavy abnormal liver functions the decrease in clearance of creatinine can display not fully extent of disturbance of functions of kidneys therefore to patients with clearance of creatinine & lt, / 1.73 sq.m a daily dose it is recommended to lower 60 ml/min. by 50%.
At children with renal failures the dose of a levetiratsetam needs to be adjusted according to degree of a renal failure.
The Clearance of Creatinine (CC) in ml/min. / 1.73 the sq.m at teenagers, children and babies can be calculated on the basis of the serumal level of creatinine (mg/dl) on the following formula (Schwartz's formula):
Growth (cm) x
ks KK (ml/min. / 1.73 m ²) =-------------------------
Creatinine of serum (mg/dl)
Ks=0.55 in case of children up to 13 years and teenage girls, in case of teenage boys of ks=0.7.
The scheme of dosing at children and teenagers with body weight is less than 50 kg with a renal failure
the Stage
Clearance of Creatinine (ml/min. / 1.73 of sq.m)
the Dose and frequency of use
Children and teenagers with
body weight less than 50 kg
Normal function of kidneys
& gt, 80
10–30 mg/kg twice a day
Insignificant extent of disturbance
of 50-79
10–20 mg/kg twice a day
Moderate extent of disturbance
of 30-49
5–15 mg/kg twice a day
Heavy extent of disturbance
& lt, 30
5–10 mg/kg twice a day
the Patients with an end-stage of a renal failure who are on dialysis
-
10–20 mg/kg
(1) (2) (1) V the first day of use of a levetiratsetam are recommended once a day reception of a load dose of 15 mg/kg.
(2) After dialysis the reception of an additional dose of 5-10 mg/kg is recommended.
Side effects
the Profile of side reactions is based on the analysis of these placebos - controlled clinical trials with coverage of 3416 patients. The nasopharyngitis, fatigue, a headache, drowsiness, dizziness were the most frequent side reactions.
The undesirable reactions given below are listed according to defeat of bodies and the systems of bodies and occurrence frequency. Frequency of occurrence is defined as follows: very often (≥ 1/10), it is frequent (≥ 1/100 and & lt, 1/10), infrequently (≥ 1/1,000 and & lt, 1/100), is rare (≥ 1/10,000 and & lt, 1/1,000), is very rare (& lt, 1/10,000).
Very often
- a nasopharyngitis
- drowsiness
- a headache
Often
- anorexia (is more often at the accompanying appointment with topiramaty)
- a depression, aggression, concern, insomnia, nervousness, irritability, emotional lability / change of mood,
- spasms, balance disturbance, dizziness, a lethargy, a tremor
- vertigo
- cough
- pain in a stomach, diarrhea, dyspepsia, nausea, vomiting
- rash
- asthenia/fatigue
Infrequently
- a leukopenia, thrombocytopenia
- decrease/increase in body weight
- attempt of a suicide, suicide thoughts, mental disorders, a behavior disorder, hallucinations, anger, confusion of consciousness, the panic attacks, emotional lability / change of mood, excitement
- amnesia, disturbance of memory, an ataxy/incoordination, paresthesia, attention disorder
- a diplopia, illegibility of sight
- pathological results of hepatic tests
- an alopecia (regression at drug withdrawal), eczema, an itching
- muscle weakness, myalgia
- injuries
Seldom
- infections
- a pancytopenia (in certain cases was followed by marrow myelosuppression), a neutropenia, an agranulocytosis
- medicinal reactions in the form of an eosinophilia and system reactions (DRESS)
- a hyponatremia
- a suicide, pathological thinking, disorder of the personality,
- a choreoathetosis, dyskinesia, a hyperkinesia
- pancreatitis
- a liver failure, hepatitis
- a toxic epidermal necrolysis, Stephens-Johnson's syndrome, a multiformny erythema
When comparing of the data obtained at treatment of 645 children aged from 4 up to 16 years it was revealed that the profile of side reactions between children and adults is similar except for behavioural and mental disorders which meet bigger frequency at children. The following side reactions meet at this cohort more often than at adults: vomiting, alarm, changes of mood, lability, aggression, atypical behavior, lethargy.
Contraindications
- hypersensitivity to a levetiratsetam, other derivatives of a pirrolidon or any auxiliary component of drug
- the children's age up to 6 years (use of drug in the form of solution for intake)
- children's and teenage age up to 18 years (for tablets with a dose of 250 mg is recommended)
Medicinal interactions
the Antiepileptic
drugs Levetiratsetam does not affect concentration of the known antiepileptic drugs in blood serum (Phenytoinum, carbamazepine, valproic acid, phenobarbital, lamotrigin, topiramat, gabapentin and Primidonum).
As well as at adults, in children's population the considerable interaction of medicines when prescribing the drug Keppra® in a dose of 60 mg/kg/days was not observed.
Assessment of indicators of pharmacokinetics at children and teenagers with epilepsy (4-17 years) confirmed that additional therapy by the drug Keppra® at intake did not affect concentration of the drugs of carbamazepine and Valproatum accepted together with levetiratsetamy. Nevertheless, the clearance of a levetiratsetam at the children receiving enzyme - the induced antiepileptic means, was 20% higher, than at children who did not take such drugs. Dose adjustment is not required.
Probenetsid
Probenetsid (blocker of renal canalicular secretion) at use on 500 mg four times a day inhibits renal clearance of primary metabolite, but not the levetiratsetam. Nevertheless, concentration of this metabolite remains low. It is expected that other medicines excreted by active tubular secretion also can reduce renal clearance of a metabolite. Influence of a levetiratsetam on pharmacokinetics of a probenetsid and other actively cosecreted drugs, such as non-steroidal anti-inflammatory drugs, sulfonamides and methotrexate is unknown.
Oral contraceptives, digoxin and warfarin
the Drug Keppra® in a daily dose of 1000 mg does not influence pharmacokinetics of oral contraceptives (ethinylestradiol and levonorgestrel).
The drug Keppra® in a daily dose of 2000 mg does not influence pharmacokinetics of digoxin and warfarin.
Digoxin, oral contraceptives and warfarin do not influence pharmacokinetics of a levetiratsetam.
Antacids
Are not present data on influence of antacids on process of absorption of a levetiratsetam.
Laxatives
At a concomitant use with an osmotic laxative macrogoal – the efficiency of a levetiratsetam decreases in this connection the macrogoal should be accepted in 1 hour prior to or 1 hour after administration of drug of Keppra®.
Food and alcohol
Meal does not influence extent of absorption of a levetiratsetam, however absorption speed slows down a little. Data on interaction of the drug Keppra® with alcohol are absent.
The special
instructions Drug Withdrawal According to the current clinical practice, in case of need drug withdrawals it it is necessary to make gradually (lowering a single dose by 500 mg each 2-4 weeks at patients with body weight more than 50 kg). At children and teenagers with body weight less than 50 kg the dose decline should not exceed 10 mg/kg of body weight twice a day each 2 weeks.
The renal or liver failure
Prescribing of the drug Keppra® to patients with a renal or liver failure can demand drug dose adjustment. At patients with heavy degree of a liver failure it is necessary to estimate functions of kidneys before therapy.
A depression / suicide thoughts
Proceeding from the received messages about suicide cases, suicide intentions and attempts of a suicide against the background of use of antiepileptic drugs, including levetiratseta, the patients and persons who are looking after them should be warned about need to report to the attending physician about emergence of any symptoms of a depression or suicide intentions. Patients have to be under medical observation regarding emergence of suicide intentions and attempts of a suicide though assessment of risk of increase in these disorders against the background of intake of antiepileptic drugs showed that degree of risk increases slightly. The origins of these risks are unknown.
Children
the Tableted form of the drug Keppra® is not recommended to use for children 6 years are younger.
The existing data on use of drug for children do not indicate any undesirable impact on development and maturing. However, the remote effects of use of drug on ability to training, intellectual development, growth, function of closed glands, puberty and fertility remain unknown.
The efficiency and safety of a levetiratsetam at children are younger than 1 year as auxiliary therapy at partial attacks with secondary generalization or without it is not established.
The efficiency and safety of a levetiratsetam as auxiliary therapy at children are younger than 12 years with juvenile myoclonic epilepsy ilis idiopathic generalized epilepsy is not established.
Pregnancy and the period of a lactation
Fertility
of the Research on animals showed lack of influence on fertility. Clinical data are absent, the potential risk for the person is unknown.
Pregnancy
In post-marketing prospective registers cases of use of drug for pregnant women in the first trimester are described. In spite of the fact that by results of these data in general increase in frequency of congenital anomalies was not traced, the teratogenic risk cannot be excluded. At co-administration of several antiepileptic drugs the risk of congenital anomalies, in communication increases with what monotherapy has to be considered.
Researches on animals showed reproductive toxicity.
It is not recommended to use the drug Keppra® at the pregnant women and women of childbearing age who are not applying contraception methods except for cases of clinical need.
As well as with other antiepileptic drugs, physiological changes during pregnancy can affect concentration of a levetiratsetam (decrease in level in blood serum). The most significant decrease is observed in the third trimester (up to 60% of initial concentration).
The women receiving to levetiratseta during pregnancy have to be provided with the corresponding clinical inspection. Breaks in treatment by antiepileptic means can lead to exacerbation of a disease that can be injurious to health of mother and a fruit.
The lactation period
Levetiratsetam is excreted in breast milk. Feeding by a breast during treatment is not recommended. However, if drug Keppra® use by nursing mothers is necessary, it is necessary to carry out the assessment of advantage and risk of reception/drug withdrawal.
The drug Keppra® is not recommended during the lactation period if there is no clinical need.
Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
Influence of the drug Keppra® on ability to drive the car or to work with mechanisms specially was not studied. However, in connection with different individual sensitivity to drug from the central nervous system, especially at the beginning of therapy or at increase in doses, it is necessary to refrain from control of motor transport and perhaps dangerous works demanding special attentiveness and speed of psychomotor reaction.
Overdose
Symptoms: drowsiness, excitement, consciousness oppression, respiratory depression, coma.
Treatment: in an acute period – an artificial call of vomiting and gastric lavage with the subsequent use of activated carbon. There is no specific antidote for a levetiratsetam. If necessary provide symptomatic treatment in clinic using a hemodialysis (efficiency of dialysis for a levetiratsetam – 60%, for its main metabolite – 74%).
Form of release and packing
of the Tablet, film coated, 250 mg, 500 mg, 1000 mg.
On 10 tablets place in blister strip packaging from a film of polyvinylchloride and aluminum foil. On 3 or 6 blister strip packagings together with the instruction for medical use in the state and Russian languages place in a cardboard pack.
To Store storage conditions in the dry place at a temperature not above 25 °C.
To store out of children's reach!
A period of storage
3 years
not to use after the termination of an expiration date
Prescription status
According to the prescription
YuSB Producer Pharma S.A., Belgium
of Allee da la Recherche 60, Brussels
the Owner of the registration certificate
GlaksoSmitKlyayn Export of Ltd., Great Britain
980 Great West Road, Brentford, Middlesex, TW8 9GS,
the UK Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods)
of Predstavitelstvo GlaksoSmitKlyayn Export Ltd in
Kazakhstan 050059, Almaty, Furmanov St., 273
Phone number: +7 727 258 28 92, +7 727 259 09 96
Fax number: + 7 727 258 28 90
E-mail address:
To Develop kaz.med@gsk.com