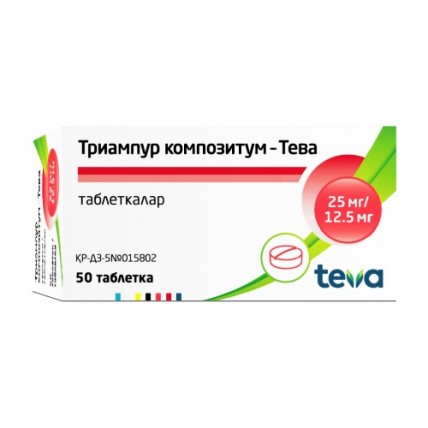
Triampur compositum® (Triamterene + Hydrochlorothiazide) 50 tablets
- $13.00
What is Triampur Compositum®?
Triampur Compositum® is a combination medication that contains two active ingredients: Triamterene (25 mg) and Hydrochlorothiazide (12.5 mg).
It is formulated in tablet form and is used to treat conditions such as high blood pressure (hypertension) and fluid retention (edema) associated with heart, liver, or kidney disorders.
The dual-action mechanism of Triampur Compositum® provides a unique balance of benefits:
- Triamterene is a potassium-sparing diuretic that helps the body eliminate excess fluid without losing too much potassium, a vital mineral needed for proper muscle and heart function.
- Hydrochlorothiazide is a thiazide diuretic that effectively removes excess sodium and water from the body, reducing swelling and controlling blood pressure.
Together, these ingredients offer a comprehensive approach to managing fluid balance and blood pressure while minimizing the risk of potassium depletion, a common side effect of many diuretics.
This medication is particularly suitable for individuals who need diuretic therapy but are at risk of electrolyte imbalances.
It can be used as part of a long-term treatment plan under medical supervision to manage chronic conditions or as a short-term solution for acute issues.
What are the benefits of Triampur Compositum®?
Triampur Compositum® offers several key health benefits, making it an effective choice for managing specific medical conditions.
This combination of Triamterene and Hydrochlorothiazide targets both symptoms and underlying issues in patients with hypertension and edema.
Below are the primary benefits:
Effective blood pressure control: By reducing the volume of fluid in the blood vessels, Triampur Compositum® helps lower blood pressure, which can reduce the risk of heart attacks, strokes, and other cardiovascular complications.
Management of edema (fluid retention): This medication effectively reduces swelling caused by fluid buildup in conditions such as heart failure, liver disease, or kidney dysfunction, helping improve overall comfort and mobility.
Potassium-sparing properties: Unlike many diuretics, Triampur Compositum® helps retain potassium, a critical electrolyte for heart and muscle function. This reduces the risk of hypokalemia (low potassium levels), a common side effect of other diuretic medications.
Dual-action mechanism: By combining Triamterene and Hydrochlorothiazide, this medicine balances fluid reduction with potassium conservation, offering a comprehensive approach to diuretic therapy.
Improved quality of life: With reduced swelling, lower blood pressure, and improved electrolyte balance, patients often experience increased energy, reduced fatigue, and better overall well-being.
Suitable for long-term use (under supervision): Triampur Compositum® is designed for both acute and chronic management of conditions when used as prescribed, ensuring consistent results over time.
How should Triampur Compositum® be used?
Dosage: For high blood pressure: Typically, 2 tablets in the morning and 2 at midday as a starting dose. Maintenance is usually 1-2 tablets per day.
For edema: Start with 2 tablets twice daily, adjusted based on the patient’s response.
Administration: Take tablets after meals with water. Do not chew the tablets.
Maintain adequate hydration during treatment.
Treatment Duration: Determined by your healthcare provider. Avoid abrupt discontinuation.
Are there precautions to consider before using this medication?
Yes, consult your doctor if you: Have kidney or liver issues. Suffer from diabetes or gout (may affect blood sugar or uric acid levels).
Are on other potassium-sparing diuretics or supplements (risk of high potassium levels).
Experience dehydration or low sodium levels.
What are the possible side effects?
Common: Dizziness, nausea, or fatigue. Rare but serious: Hyperkalemia (high potassium levels), low blood pressure, or allergic reactions like rash or swelling.
Long-term use concerns: Slight increase in cholesterol or risk of skin cancer with prolonged exposure to sunlight.
How to store Triampur Compositum®?
Store at temperatures below 25°C (77°F).
Keep out of reach of children. Do not use past the expiration date.