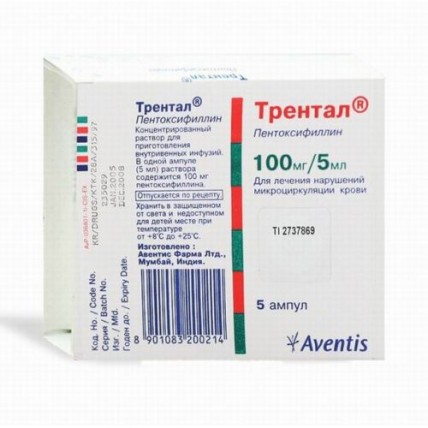
Trental 100 mg / 5 ml 5's solution for injection in ampoules
- $23.00
Out Of Stock
The instruction for medical use
of Trental medicine
the Trade name
of Trental
the International unlicensed
name Pentoksifillin Lekarstvennaya a form
the Concentrate for preparation of solution for infusions of 20 mg/ml
Structure
of 1 ml of solution contains
active agent - pentoksifillin 20.00 mg,
excipients: sodium chloride, water for injections.
Description
Transparent, colourless or almost colourless solution.
Pharmacotherapeutic group
Peripheral vazodilatator. Purines. Pentoksifillin.
The ATX C04AD03 code
the Pharmacological
Pharmacokinetics At properties intravenous administration pentoksifillin comes directly to a blood stream, at the same time the maximum concentration it in blood is practically at once reached and the maximum effect develops. The volume of distribution reaches average values of 280-465 liters at bolyusny introduction by doses of 25-100 mg. Linking of a pentoksifillin with human albumine is not revealed.
Pentoksifillin is metabolized substantially both after peroral and after intravenous administration. This process begins soon after introduction and has properties of saturable metabolism of primary passing. The chemical structure of 7 products of biotransformation (MI-MVII) pentoksifillin is revealed now. The main metabolites – MV, MI, MVII – are found in blood soon after introduction. MV reaches the highest concentrations. Elimination half-life of initial connection and its three metabolites varies from 0.5 to 1.5 hours. Rheological researches showed what is possible to characterize almost equally important pharmacological metabolites of I, IV and V as initial connection.
Elimination half-life of a pentoksifillin after intravenous administration makes, about 1.6 hours. About 90%-95% of substance are removed by kidneys in the form of metabolites and about 5% with a stake.
Special group of patients
the Abnormal liver function
At patients with an abnormal liver function the elimination half-life of a pentoksifillin is extended, and the absolute bioavailability is increased.
The renal failure
Removal of metabolites is slowed down at patients with a heavy renal failure.
The pharmacodynamics
of Trental increases the broken deformability of erythrocytes, reduces aggregation of erythrocytes, thrombocytes, reduces the level of fibrinogen and adhesion of leukocytes to an endothelium, reduces activation of leukocytes and the damage of an endothelium caused by it, reduces viscosity of blood. Therefore, Trental promotes microcirculator perfusion, by improvement of fluidity of blood and rendering antitrombotichesky effects. Peripheric resistance can decrease slightly if Trental is entered in high doses or in the form of fast infusion. Trental renders moderate positive inotropic effect on heart.
Indications
- the occlusal disease of peripheral arteries (ODPA) of arteriosclerotic or diabetic genesis (for example, the alternating lameness or pains at rest)
- trophic defeats (for example, ulcers and gangrene of legs)
- vascular diseases of a brain (cerebrovascular diseases)
- disturbances of blood circulation in a retina and a choroid of an eye in connection with degenerative vascular disorders
the Route of administration and doses
the Dosage and a method of administration (peroral or intravenous) depend on type and weight of disturbances of blood circulation and individual medicinal shipping by the patient. As a rule, the dose is selected, being guided by the following principles.
The II stage of OBPA (the alternating lameness) and disturbance of blood circulation in a retina and a choroid of an eye: the initiation of treatment or support of oral therapy
Infusion of 100 mg - 600 mg of Trental is recommended to enter one or two times a day. Infusion of Trental is recommended to be entered in the corresponding infusion solution, depending on associated diseases (for example, stagnant heart failure) reduction of the entered volume of infusions can be required. In such cases it is necessary to use an infusional pomp with a controlled volume.
If infusional therapy by low doses is combined with oral therapy, the recommended general daily dose makes 1200 mg of Trental (intravenously + orally).
The subsequent therapy is recommended to be continued only by the tableted form of a pentoxyeagle owl (orally).
III and IV stages of OBPA
the General daily dose of 1200 mg of Trental are recommended to be entered in the corresponding solution carrier or as continuous infusion for 24 hours or as the infusion of 600 mg entered twice a day during not less than six-hour periods.
Depending on associated diseases (for example, stagnant heart failure) reduction of the entered volume of infusions can be required. In such cases the infusional pomp with a controlled volume is necessary. As for the subsequent treatment, therapy can be continued by one tableted form of a pentoksifillin (orally).
Special groups of patients
Use in
Experience pediatrics on use for children is not present.
The abnormal liver function
the Dose decline depending on individual shipping is necessary in case of patients with a heavy abnormal liver function.
The renal failure
can be required by Patients with a renal failure (the clearance of creatinine is lower than 30 ml/min.) a dose decline approximately for 30-50% depending on individual shipping.
Other
Treatment needs to be begun with small doses at patients with hypotension and patients with unstable blood circulation and also the patients subject to extra risk because of a lowering of arterial pressure (for example, patients with heavy coronary heart disease or the profound stenoses of the blood vessels supplying a brain), in such cases have to have a gradual increase in a dose.
Introduction
is valid only for oral forms
the Usual dose makes 400 mg of Trental on 2-3 times a day or 600 mg twice a day.
Really for the dosage forms intended for parenteral administration
Duration of infusion has to be not less than 60 minutes on 100 mg of Trental.
Mutual influence on laboratory and diagnostic tests
Is not present data.
Abuse and dependence
is not applicable.
Side effects
Frequency is unknown
- dizziness, a headache, aseptic meningitis
- agitation, a sleep disorder
- an itching, an erythema, urticaria
- inflows of heat, bleeding
- arrhythmia, tachycardia, stenocardia
- gastrointestinal disturbances, discomfort in epigastric area, the abdominal distension, nausea, vomiting, diarrhea
- a cholestasia (intra hepatic)
- increase in transaminases, a lowering of arterial pressure
- thrombocytopenia
- anaphylactic reaction, anaphylactoid reaction, a Quincke's disease, a bronchospasm, an acute anaphylaxis
of the Contraindication
- hypersensitivity to a pentoksifillin, other methylxanthines or to any of excipients
- patients with massive bleeding (threat of strengthening of bleeding)
- extensive retinal apoplexies of an eye (threat of strengthening of bleeding)
- children's and teenage age up to 18 years
Medicinal interactions
Glyukozoponizhayushchy effect of insulin and other oral antidiabetic means in blood can amplify. Therefore, it is recommended to control carefully the patients who are on drug treatment concerning diabetes.
Cases of increase in anticoagulative activity from post-marketing practice were registered at the patients who were at the same time treated pentoksifilliny and vitamin K inhibitors. In case of these patients when begin treatment with Trental or change its dose, it is recommended to conduct monitoring of anticoagulative activity.
It is necessary to take into account
of Trental can enhance hypotensive effect of the antihypertensive drugs and other connections capable to have hypotensive effect. Simultaneous administration of Trental with theophylline can increase concentration of theophylline at some patients. Therefore, strengthening and increase of undesirable reactions to theophylline is possible.
Special indications
of the Precautionary measure
At the first signs of anaphylactic (anaphylactoid) reaction intake of Trental it is necessary to stop and inform the attending physician.
Especially careful monitoring is required from patients
- with a cerebral hemorrhage
- with a proliferating diabetic retinopathy
- with heavy cardiac arrhythmias
- with a myocardial infarction
- with hypotension
- with disturbance of renal function (clearance of creatinine less than 30 ml/min.)
- with a heavy abnormal liver function
- with the raised tendency to bleeding (see also Contraindications)
- being at the same time on treatment pentoksifilliny and vitamin K inhibitors (see also Medicinal interactions)
- being at the same time on treatment pentoksifilliny and antidiabetic means (see also Medicinal interactions)
- being in risk group in view of a possible lowering of arterial pressure (for example, patients with a severe form of an ischemic heart disease or a significant stenosis of the blood vessels supplying a brain).
Use in
Experience pediatrics on use of Trental for children is not present therefore its appointment at children's and teenage age up to 18 years is not recommended.
Pregnancy and a lactation
Experience on use during pregnancy is insufficient therefore use of Trental during pregnancy is not recommended.
Pentoksifillin gets into breast milk in insignificant quantities. In view of lack of sufficient experience, doctors should weigh carefully possible risk and advantage before appointing treatment Trental to the women nursing.
Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
it is not applicable.
Overdose
Symptoms: Nausea, dizziness, tachycardia or falling of arterial blood pressure can be initial symptoms of acute overdose by Trental. Besides, such symptoms as high temperature of a body, agitation, rushes of blood, a loss of consciousness, an areflexia, tonoclonic convulsions and as symptom of gastrointestinal bleeding, a coffee-ground vomit are possible.
Treatment: Symptomatic. Specific antidote is unknown.
A form of release and packing
On 5 ml of drug in ampoules of colourless glass with a blue dot (line of a break) above a neck. On an ampoule paste the label from paper label.
On 5 ampoules together with the instruction for medical use in the state and Russian languages put in a cardboard pack.
To Store storage conditions in the place protected from light at a temperature from 8 wasps up to 25 wasps.
To store out of children's reach!
4 years
not to apply a period of storage after an expiration date.
Prescription status
According to the prescription
the Producer
of Sanofi India Limited, India
the location Address: 54/A of Sir Mathuradas Road, Andheri (E), Mumbai-400,093, India
the Owner of the registration certificate
of Sanofi India Limited, India
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods)
050013, Almaty, Furmanov St. 187B
phone number: +7 (727) 244-50-96
fax: +7 (727) 258-25-96
e-mail:
To Develop quality.info@sanofi.com
of Trental medicine
the Trade name
of Trental
the International unlicensed
name Pentoksifillin Lekarstvennaya a form
the Concentrate for preparation of solution for infusions of 20 mg/ml
Structure
of 1 ml of solution contains
active agent - pentoksifillin 20.00 mg,
excipients: sodium chloride, water for injections.
Description
Transparent, colourless or almost colourless solution.
Pharmacotherapeutic group
Peripheral vazodilatator. Purines. Pentoksifillin.
The ATX C04AD03 code
the Pharmacological
Pharmacokinetics At properties intravenous administration pentoksifillin comes directly to a blood stream, at the same time the maximum concentration it in blood is practically at once reached and the maximum effect develops. The volume of distribution reaches average values of 280-465 liters at bolyusny introduction by doses of 25-100 mg. Linking of a pentoksifillin with human albumine is not revealed.
Pentoksifillin is metabolized substantially both after peroral and after intravenous administration. This process begins soon after introduction and has properties of saturable metabolism of primary passing. The chemical structure of 7 products of biotransformation (MI-MVII) pentoksifillin is revealed now. The main metabolites – MV, MI, MVII – are found in blood soon after introduction. MV reaches the highest concentrations. Elimination half-life of initial connection and its three metabolites varies from 0.5 to 1.5 hours. Rheological researches showed what is possible to characterize almost equally important pharmacological metabolites of I, IV and V as initial connection.
Elimination half-life of a pentoksifillin after intravenous administration makes, about 1.6 hours. About 90%-95% of substance are removed by kidneys in the form of metabolites and about 5% with a stake.
Special group of patients
the Abnormal liver function
At patients with an abnormal liver function the elimination half-life of a pentoksifillin is extended, and the absolute bioavailability is increased.
The renal failure
Removal of metabolites is slowed down at patients with a heavy renal failure.
The pharmacodynamics
of Trental increases the broken deformability of erythrocytes, reduces aggregation of erythrocytes, thrombocytes, reduces the level of fibrinogen and adhesion of leukocytes to an endothelium, reduces activation of leukocytes and the damage of an endothelium caused by it, reduces viscosity of blood. Therefore, Trental promotes microcirculator perfusion, by improvement of fluidity of blood and rendering antitrombotichesky effects. Peripheric resistance can decrease slightly if Trental is entered in high doses or in the form of fast infusion. Trental renders moderate positive inotropic effect on heart.
Indications
- the occlusal disease of peripheral arteries (ODPA) of arteriosclerotic or diabetic genesis (for example, the alternating lameness or pains at rest)
- trophic defeats (for example, ulcers and gangrene of legs)
- vascular diseases of a brain (cerebrovascular diseases)
- disturbances of blood circulation in a retina and a choroid of an eye in connection with degenerative vascular disorders
the Route of administration and doses
the Dosage and a method of administration (peroral or intravenous) depend on type and weight of disturbances of blood circulation and individual medicinal shipping by the patient. As a rule, the dose is selected, being guided by the following principles.
The II stage of OBPA (the alternating lameness) and disturbance of blood circulation in a retina and a choroid of an eye: the initiation of treatment or support of oral therapy
Infusion of 100 mg - 600 mg of Trental is recommended to enter one or two times a day. Infusion of Trental is recommended to be entered in the corresponding infusion solution, depending on associated diseases (for example, stagnant heart failure) reduction of the entered volume of infusions can be required. In such cases it is necessary to use an infusional pomp with a controlled volume.
If infusional therapy by low doses is combined with oral therapy, the recommended general daily dose makes 1200 mg of Trental (intravenously + orally).
The subsequent therapy is recommended to be continued only by the tableted form of a pentoxyeagle owl (orally).
III and IV stages of OBPA
the General daily dose of 1200 mg of Trental are recommended to be entered in the corresponding solution carrier or as continuous infusion for 24 hours or as the infusion of 600 mg entered twice a day during not less than six-hour periods.
Depending on associated diseases (for example, stagnant heart failure) reduction of the entered volume of infusions can be required. In such cases the infusional pomp with a controlled volume is necessary. As for the subsequent treatment, therapy can be continued by one tableted form of a pentoksifillin (orally).
Special groups of patients
Use in
Experience pediatrics on use for children is not present.
The abnormal liver function
the Dose decline depending on individual shipping is necessary in case of patients with a heavy abnormal liver function.
The renal failure
can be required by Patients with a renal failure (the clearance of creatinine is lower than 30 ml/min.) a dose decline approximately for 30-50% depending on individual shipping.
Other
Treatment needs to be begun with small doses at patients with hypotension and patients with unstable blood circulation and also the patients subject to extra risk because of a lowering of arterial pressure (for example, patients with heavy coronary heart disease or the profound stenoses of the blood vessels supplying a brain), in such cases have to have a gradual increase in a dose.
Introduction
is valid only for oral forms
the Usual dose makes 400 mg of Trental on 2-3 times a day or 600 mg twice a day.
Really for the dosage forms intended for parenteral administration
Duration of infusion has to be not less than 60 minutes on 100 mg of Trental.
Mutual influence on laboratory and diagnostic tests
Is not present data.
Abuse and dependence
is not applicable.
Side effects
Frequency is unknown
- dizziness, a headache, aseptic meningitis
- agitation, a sleep disorder
- an itching, an erythema, urticaria
- inflows of heat, bleeding
- arrhythmia, tachycardia, stenocardia
- gastrointestinal disturbances, discomfort in epigastric area, the abdominal distension, nausea, vomiting, diarrhea
- a cholestasia (intra hepatic)
- increase in transaminases, a lowering of arterial pressure
- thrombocytopenia
- anaphylactic reaction, anaphylactoid reaction, a Quincke's disease, a bronchospasm, an acute anaphylaxis
of the Contraindication
- hypersensitivity to a pentoksifillin, other methylxanthines or to any of excipients
- patients with massive bleeding (threat of strengthening of bleeding)
- extensive retinal apoplexies of an eye (threat of strengthening of bleeding)
- children's and teenage age up to 18 years
Medicinal interactions
Glyukozoponizhayushchy effect of insulin and other oral antidiabetic means in blood can amplify. Therefore, it is recommended to control carefully the patients who are on drug treatment concerning diabetes.
Cases of increase in anticoagulative activity from post-marketing practice were registered at the patients who were at the same time treated pentoksifilliny and vitamin K inhibitors. In case of these patients when begin treatment with Trental or change its dose, it is recommended to conduct monitoring of anticoagulative activity.
It is necessary to take into account
of Trental can enhance hypotensive effect of the antihypertensive drugs and other connections capable to have hypotensive effect. Simultaneous administration of Trental with theophylline can increase concentration of theophylline at some patients. Therefore, strengthening and increase of undesirable reactions to theophylline is possible.
Special indications
of the Precautionary measure
At the first signs of anaphylactic (anaphylactoid) reaction intake of Trental it is necessary to stop and inform the attending physician.
Especially careful monitoring is required from patients
- with a cerebral hemorrhage
- with a proliferating diabetic retinopathy
- with heavy cardiac arrhythmias
- with a myocardial infarction
- with hypotension
- with disturbance of renal function (clearance of creatinine less than 30 ml/min.)
- with a heavy abnormal liver function
- with the raised tendency to bleeding (see also Contraindications)
- being at the same time on treatment pentoksifilliny and vitamin K inhibitors (see also Medicinal interactions)
- being at the same time on treatment pentoksifilliny and antidiabetic means (see also Medicinal interactions)
- being in risk group in view of a possible lowering of arterial pressure (for example, patients with a severe form of an ischemic heart disease or a significant stenosis of the blood vessels supplying a brain).
Use in
Experience pediatrics on use of Trental for children is not present therefore its appointment at children's and teenage age up to 18 years is not recommended.
Pregnancy and a lactation
Experience on use during pregnancy is insufficient therefore use of Trental during pregnancy is not recommended.
Pentoksifillin gets into breast milk in insignificant quantities. In view of lack of sufficient experience, doctors should weigh carefully possible risk and advantage before appointing treatment Trental to the women nursing.
Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
it is not applicable.
Overdose
Symptoms: Nausea, dizziness, tachycardia or falling of arterial blood pressure can be initial symptoms of acute overdose by Trental. Besides, such symptoms as high temperature of a body, agitation, rushes of blood, a loss of consciousness, an areflexia, tonoclonic convulsions and as symptom of gastrointestinal bleeding, a coffee-ground vomit are possible.
Treatment: Symptomatic. Specific antidote is unknown.
A form of release and packing
On 5 ml of drug in ampoules of colourless glass with a blue dot (line of a break) above a neck. On an ampoule paste the label from paper label.
On 5 ampoules together with the instruction for medical use in the state and Russian languages put in a cardboard pack.
To Store storage conditions in the place protected from light at a temperature from 8 wasps up to 25 wasps.
To store out of children's reach!
4 years
not to apply a period of storage after an expiration date.
Prescription status
According to the prescription
the Producer
of Sanofi India Limited, India
the location Address: 54/A of Sir Mathuradas Road, Andheri (E), Mumbai-400,093, India
the Owner of the registration certificate
of Sanofi India Limited, India
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods)
050013, Almaty, Furmanov St. 187B
phone number: +7 (727) 244-50-96
fax: +7 (727) 258-25-96
e-mail:
To Develop quality.info@sanofi.com