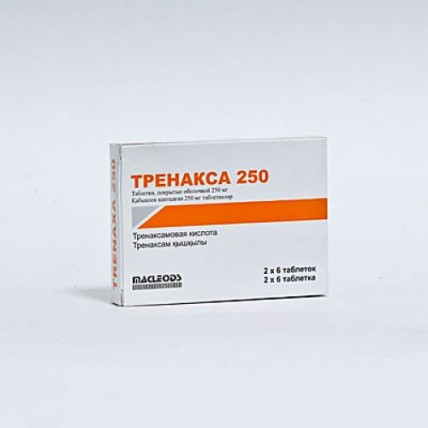
Trenaxa (Tranexamic Acid) 250 mg, 12 coated tablets
- $16.10
The instruction
for medical use
of TPEHAKCA 250 medicine
TPEHAKCA 500
the Trade name
of Trenaks 250
Trenaks 500
International unlicensed
name Traneksamovaya acid
the Dosage form
of the Tablet, coated, 250 mg or 500 mg
Structure
One tablet contains
active agent - acid of traneksamovy 250 mg or 500 mg,
excipients: cellulose microcrystalline (PH 101), povidone (PVP–K-30), cellulose microcrystalline (PH 302), sodium of a kroskarmelloz, silicon colloidal anhydrous, the talc purified magnesium stearate,
a cover: a gipromelloza (5 cps), the titan dioxide (E 171), the talc purified, propylene glycol, diethyl phthalate.
The description
of the Tablet of round shape with a biconvex surface, film coated white color, smooth on both sides (for a dosage of 250 mg).
Tablets of round shape with a biconvex surface, film coated white color, with risky on one party and smooth with another (for a dosage of 500 mg).
Pharmacotherapeutic group
of Gemostatiki. Amino acids. Traneksamovy acid
ATX B02AA02 Code
Pharmacological
Pharmacokinetics Absorption properties: after intake from 30 to 50% of the traneksamovy acid (TA) are soaked up. The maximum concentration in plasma is reached in 2.17 hours after reception of 500 mg of drug and makes 1.45 mkg/ml. Meal does not reduce extent of absorption in a stomach and does not change other pharmacokinetic indicators of shopping mall.
Distribution: traneksamovy acid easily gets through hematoencephalic and placentary barriers, initial volume of distribution – 9-12 l. In synovial membranes and intra articulate liquid collects in the concentration close to serumal. Concentration in many other fabrics is lower, than in blood. In cerebrospinal liquid the concentration of shopping mall makes about 10% of that in plasma. The shopping mall is found in semen where suppresses fibrinolytic activity of blood, but does not affect mobility of spermatozoa. Linking with plasminogen - 2-3% of therapeutic concentration in plasma. Does not contact plasma albumine. In plasma remains within 4.52 hours. The bioavailability does not depend on meal.
Metabolism and removal: the main way of removal - glomerular filtration. More than 95% are removed with urine in not changed look. An insignificant part is exposed to biotransformation: The N-acetylized derivative and dezaminirovanny dekarboksilny acid are found in urine in insignificant quantities.
After intake of traneksamovy acid in a dose of 10-15 mg/kg a day, cumulative renal excretion in 24 and 48 hours is 39% and 41%, respectively, or 78% and 82% of the absorbed substance. After intake the metabolites are found in quantity: 1% of dicarboxyl acid and 0.5% of an acetylized metabolite.
The pharmacodynamics
of Traneksamovaya acid has anti-fibrinolytic effect.
Action mechanism: competitively inhibits plasminogen activator, in higher concentrations of shopping mall is not a competitive inhibitor of plasmin.
The inhibiting effect of traneksamovy acid on plasminogen activation by an urokinase and streptokinase by 6-100 times and 6-40 times respectively is more, than at aminocapronic acid. The anti-fibrinolytic activity of traneksamovy acid is approximately ten times more, than at aminocapronic acid.
Indications
For short-term use in bleeding or risk of bleeding for patients with the raised fibrinolysis or fibrinogenolizy.
A local fibrinolysis which occurs in the following cases:
- prostatectomy, bladder surgeries
- conization of a neck of the uterus
- a menorrhagia
- nasal bleedings
- a traumatic hyphema
- extraction of teeth at patients with hemophilia
- a hereditary Quincke's disease
the Route of administration and doses
the dosing Mode individual, depending on a clinical situation. A pill is taken inside regardless of meal, without chewing, washing down with enough water.
Local fibrinolysis
the Recommended standard dose makes 15-25¼ú/kg body weights (2-3 tablets Trenaksa of 500 or 4-6 tablets Trenaksa 250) 2-3 times a day.
For the indications which are listed below the following dosages can be used:
Prostatectomy
Prevention and treatment of bleedings at patients of high risk should be begun to or after operation with the help of an injection of traneksamovy acid,
then to continue reception of 2 tablets 3-4 times a day before disappearance of a macroscopic hamaturia
the Menorrhagia
the Recommended dose makes 2 tablets Trenaksa 500 or 4 tablets Trenaksa 250 three times a day with reception duration depending on need for a period of up to 4 days. In very plentiful menstrual bleedings the dose can be increased. The general dose should not exceed 4 g daily (8 tablets Trenaksa of 500 or 16 tablets Trenaksa 250). Treatment by traneksamovy acid should not be begun prior to the beginning of periods.
Nasal bleeding
In repeated bleeding is appointed oral therapy (2 tablets Trenaksa 500 or 4 tablets Trenaksa 250 three times a day) within 7 days.
Conization of a neck of the uterus
3 tablets Trenaksa of 500 or 6 tablets Trenaksa 250 rub once a day.
A traumatic hyphema
2-3 tablets Trenaksa of 500 or 4-6 tablets Trenaksa 250 rub once a day. The dose is based at the rate of 25 mg/kg 3 times a day.
Hemophilia
At odontectomy 2-3 tablets Trenaksa of 500 or 4-6 tablets Trenaksa 250 each eight hours. The dose is based at the rate of 25 mg/kg 3 times a day.
Some patients know a hereditary Quincke's disease about the beginning of a disease, the recommended treatment for such patients - periodic reception of 2-3 tablets Trenaksa of 500 or 4-6 tablets Trenaksa 250 2-3 times a day within several days. Other patients accept treatment constantly at this dosage.
Children
of 20-25 mg/kg of body weight a day inside. Nevertheless, data on use, efficiency and safety of these indications are limited.
Elderly
Reduction of a dosage is not required if there are no symptoms of a renal failure.
The renal failure
of Traneksamovaya acid is removed with urine in not changed look therefore for patients with a renal failure smaller doses, than usually are recommended. The following dose adjustment is necessary for intake:
Plasma creatinine
the Dose
of 120-249
µmol/l 15 mg/kg of body weight 2 times in day
of 250-500
µmol/l 15 mg/kg of body weight of 1 times a day
Side effects
Frequency is defined as: very often (= 1/l0), it is frequent (= 1/100 to & lt, 1/10), infrequently (= 1/1000 to & lt, 1/100), is rare (= 1/10, 000 to & lt, 1/1000) and is very rare (& lt, 1/10, 000), it is not known (it cannot be estimated from the available data).
Seldom
- skin allergic reactions
- disturbance of color sight
- occlusion of veins and arteries of a retina
- tromboembolic episodes
Very seldom
- reactions of hypersensitivity, including an acute anaphylaxis
- an indisposition, weakness, dizziness, drowsiness, spasms
- tachycardia, thorax pain, hypotension with a possible loss of consciousness
- thrombosis of arteries and veins
- the dispeptic phenomena: nausea, vomiting, diarrhea,
Contraindication anorexia
- hypersensitivity to traneksamovy acid or any other ingredient of drug
- a clotting disease
- thromboses of vein or arteries in the anamnesis
- a fibrinolytic state after a consumption coagulopathy
- subarachnoidal hemorrhage
- a myocardial infarction
- a heavy renal failure
- spasms in the anamnesis
- disturbance of color sight
Medicinal interactions
of Traneksamovaya acid weakens thrombolytic effect of fibrinolytic drugs.
At simultaneous use with haemo static drugs and a haemo coagualase the activation of a thrombogenesis is possible. The dezoksiepinefrina a hydrochloride, a metarmina a bitartrate, Dipiridamolum, diazepam is incompatible with an urokinase, noradrenaline a bitartrate.
Special instructions
At long-term treatment of patients with a hereditary Quincke's disease the regular observation of the ophthalmologist with check of sharpness, fields and color sight, intraocular pressure, survey of an eyeground is necessary.
With care apply in combination with fabric haemo static drugs, a haemo coagualase (in high doses), heparin.
At treatment of a hamaturia of renal genesis the risk of a mechanical anury as a result of formation of a clot increases in an ureter.
Patients with irregular menstrual bleedings should not use traneksamovy acid before establishment of the cause of irregular bleedings. If menstrual bleedings inadequately go down at intake of traneksamovy acid, it is necessary to consider alternative treatment. Patients with a thromboembolic disease in the anamnesis, including in the family anamnesis (patients with a thrombophilia), have to use traneksamovy acid only on strict medical indications and under strict observation of the doctor. Use of traneksamovy acid in cases of the raised fibrinolysis owing to disseminate intravascular fibrillation is not recommended. Clinical experience of use of traneksamovy acid for children with a menorrhagia aged to 15 years is absent.
In a renal failure (depending on extent of increase in creatinine of serum) reduce a dose and frequency rate of introduction.
Before use of traneksamovy acid it is necessary to investigate risk factors of thromboembolic diseases. The patients having a disorder of vision have to stop treatment. The patients accepting oral contraceptives because of the increased risk of developing thrombosis should appoint Traneksamovy acid with care.
Pregnancy and the period of a lactation
of Traneksamovaya acid gets through a placenta. In researches on animals there are no proofs that traneksamovy acid has teratogenic effect, however pregnant women should use drug with care. Traneksamovy acid gets into breast milk in concentration about one cell from concentration in mother's blood. The anti-fibrinolytic effect at the baby is improbable. However it is necessary to use with care drug at the women nursing.
Features of influence of medicine on ability to run the vehicle and potentially dangerous mechanisms
Considering side effects of medicine it is necessary to be careful at control of motor transport and potentially dangerous mechanisms.
Overdose
Symptoms: nausea, vomiting or orthostatic hypotension.
Treatment: gastric lavage, activated carbon, plentiful drink, symptomatic, perhaps anticoagulating treatment.
The form of release and packing
On 6 tablets place in planimetric bezjyacheykovy packing from aluminum foil.
On 2 packs together with the instruction for medical use in the state and Russian languages put in a cardboard pack.
To Store storage conditions in the dry, protected from light place at a temperature not above 25 °C.
To store out of children's reach!
A period of storage
2 years
not to use after an expiration date
Prescription status
According to the prescription
the Name and the country
of the Macleods Pharmaceuticals Limited manufacturing organization, India
304, Atlanta Arcade, Marol Church Road, Andheri (East), Mumbai – 400,059, India.
The owner of the registration certificate
of Macleods Pharmaceuticals Limited, India
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products Branch KOO "Macleods Pharmaceuticals Limited" RK, Almaty, Tulebayev St. 38, office 307 Ph./fax. +7 727 2734593, 2441294, E-mail:
To Develop danielt@macleodspharma.com
for medical use
of TPEHAKCA 250 medicine
TPEHAKCA 500
the Trade name
of Trenaks 250
Trenaks 500
International unlicensed
name Traneksamovaya acid
the Dosage form
of the Tablet, coated, 250 mg or 500 mg
Structure
One tablet contains
active agent - acid of traneksamovy 250 mg or 500 mg,
excipients: cellulose microcrystalline (PH 101), povidone (PVP–K-30), cellulose microcrystalline (PH 302), sodium of a kroskarmelloz, silicon colloidal anhydrous, the talc purified magnesium stearate,
a cover: a gipromelloza (5 cps), the titan dioxide (E 171), the talc purified, propylene glycol, diethyl phthalate.
The description
of the Tablet of round shape with a biconvex surface, film coated white color, smooth on both sides (for a dosage of 250 mg).
Tablets of round shape with a biconvex surface, film coated white color, with risky on one party and smooth with another (for a dosage of 500 mg).
Pharmacotherapeutic group
of Gemostatiki. Amino acids. Traneksamovy acid
ATX B02AA02 Code
Pharmacological
Pharmacokinetics Absorption properties: after intake from 30 to 50% of the traneksamovy acid (TA) are soaked up. The maximum concentration in plasma is reached in 2.17 hours after reception of 500 mg of drug and makes 1.45 mkg/ml. Meal does not reduce extent of absorption in a stomach and does not change other pharmacokinetic indicators of shopping mall.
Distribution: traneksamovy acid easily gets through hematoencephalic and placentary barriers, initial volume of distribution – 9-12 l. In synovial membranes and intra articulate liquid collects in the concentration close to serumal. Concentration in many other fabrics is lower, than in blood. In cerebrospinal liquid the concentration of shopping mall makes about 10% of that in plasma. The shopping mall is found in semen where suppresses fibrinolytic activity of blood, but does not affect mobility of spermatozoa. Linking with plasminogen - 2-3% of therapeutic concentration in plasma. Does not contact plasma albumine. In plasma remains within 4.52 hours. The bioavailability does not depend on meal.
Metabolism and removal: the main way of removal - glomerular filtration. More than 95% are removed with urine in not changed look. An insignificant part is exposed to biotransformation: The N-acetylized derivative and dezaminirovanny dekarboksilny acid are found in urine in insignificant quantities.
After intake of traneksamovy acid in a dose of 10-15 mg/kg a day, cumulative renal excretion in 24 and 48 hours is 39% and 41%, respectively, or 78% and 82% of the absorbed substance. After intake the metabolites are found in quantity: 1% of dicarboxyl acid and 0.5% of an acetylized metabolite.
The pharmacodynamics
of Traneksamovaya acid has anti-fibrinolytic effect.
Action mechanism: competitively inhibits plasminogen activator, in higher concentrations of shopping mall is not a competitive inhibitor of plasmin.
The inhibiting effect of traneksamovy acid on plasminogen activation by an urokinase and streptokinase by 6-100 times and 6-40 times respectively is more, than at aminocapronic acid. The anti-fibrinolytic activity of traneksamovy acid is approximately ten times more, than at aminocapronic acid.
Indications
For short-term use in bleeding or risk of bleeding for patients with the raised fibrinolysis or fibrinogenolizy.
A local fibrinolysis which occurs in the following cases:
- prostatectomy, bladder surgeries
- conization of a neck of the uterus
- a menorrhagia
- nasal bleedings
- a traumatic hyphema
- extraction of teeth at patients with hemophilia
- a hereditary Quincke's disease
the Route of administration and doses
the dosing Mode individual, depending on a clinical situation. A pill is taken inside regardless of meal, without chewing, washing down with enough water.
Local fibrinolysis
the Recommended standard dose makes 15-25¼ú/kg body weights (2-3 tablets Trenaksa of 500 or 4-6 tablets Trenaksa 250) 2-3 times a day.
For the indications which are listed below the following dosages can be used:
Prostatectomy
Prevention and treatment of bleedings at patients of high risk should be begun to or after operation with the help of an injection of traneksamovy acid,
then to continue reception of 2 tablets 3-4 times a day before disappearance of a macroscopic hamaturia
the Menorrhagia
the Recommended dose makes 2 tablets Trenaksa 500 or 4 tablets Trenaksa 250 three times a day with reception duration depending on need for a period of up to 4 days. In very plentiful menstrual bleedings the dose can be increased. The general dose should not exceed 4 g daily (8 tablets Trenaksa of 500 or 16 tablets Trenaksa 250). Treatment by traneksamovy acid should not be begun prior to the beginning of periods.
Nasal bleeding
In repeated bleeding is appointed oral therapy (2 tablets Trenaksa 500 or 4 tablets Trenaksa 250 three times a day) within 7 days.
Conization of a neck of the uterus
3 tablets Trenaksa of 500 or 6 tablets Trenaksa 250 rub once a day.
A traumatic hyphema
2-3 tablets Trenaksa of 500 or 4-6 tablets Trenaksa 250 rub once a day. The dose is based at the rate of 25 mg/kg 3 times a day.
Hemophilia
At odontectomy 2-3 tablets Trenaksa of 500 or 4-6 tablets Trenaksa 250 each eight hours. The dose is based at the rate of 25 mg/kg 3 times a day.
Some patients know a hereditary Quincke's disease about the beginning of a disease, the recommended treatment for such patients - periodic reception of 2-3 tablets Trenaksa of 500 or 4-6 tablets Trenaksa 250 2-3 times a day within several days. Other patients accept treatment constantly at this dosage.
Children
of 20-25 mg/kg of body weight a day inside. Nevertheless, data on use, efficiency and safety of these indications are limited.
Elderly
Reduction of a dosage is not required if there are no symptoms of a renal failure.
The renal failure
of Traneksamovaya acid is removed with urine in not changed look therefore for patients with a renal failure smaller doses, than usually are recommended. The following dose adjustment is necessary for intake:
Plasma creatinine
the Dose
of 120-249
µmol/l 15 mg/kg of body weight 2 times in day
of 250-500
µmol/l 15 mg/kg of body weight of 1 times a day
Side effects
Frequency is defined as: very often (= 1/l0), it is frequent (= 1/100 to & lt, 1/10), infrequently (= 1/1000 to & lt, 1/100), is rare (= 1/10, 000 to & lt, 1/1000) and is very rare (& lt, 1/10, 000), it is not known (it cannot be estimated from the available data).
Seldom
- skin allergic reactions
- disturbance of color sight
- occlusion of veins and arteries of a retina
- tromboembolic episodes
Very seldom
- reactions of hypersensitivity, including an acute anaphylaxis
- an indisposition, weakness, dizziness, drowsiness, spasms
- tachycardia, thorax pain, hypotension with a possible loss of consciousness
- thrombosis of arteries and veins
- the dispeptic phenomena: nausea, vomiting, diarrhea,
Contraindication anorexia
- hypersensitivity to traneksamovy acid or any other ingredient of drug
- a clotting disease
- thromboses of vein or arteries in the anamnesis
- a fibrinolytic state after a consumption coagulopathy
- subarachnoidal hemorrhage
- a myocardial infarction
- a heavy renal failure
- spasms in the anamnesis
- disturbance of color sight
Medicinal interactions
of Traneksamovaya acid weakens thrombolytic effect of fibrinolytic drugs.
At simultaneous use with haemo static drugs and a haemo coagualase the activation of a thrombogenesis is possible. The dezoksiepinefrina a hydrochloride, a metarmina a bitartrate, Dipiridamolum, diazepam is incompatible with an urokinase, noradrenaline a bitartrate.
Special instructions
At long-term treatment of patients with a hereditary Quincke's disease the regular observation of the ophthalmologist with check of sharpness, fields and color sight, intraocular pressure, survey of an eyeground is necessary.
With care apply in combination with fabric haemo static drugs, a haemo coagualase (in high doses), heparin.
At treatment of a hamaturia of renal genesis the risk of a mechanical anury as a result of formation of a clot increases in an ureter.
Patients with irregular menstrual bleedings should not use traneksamovy acid before establishment of the cause of irregular bleedings. If menstrual bleedings inadequately go down at intake of traneksamovy acid, it is necessary to consider alternative treatment. Patients with a thromboembolic disease in the anamnesis, including in the family anamnesis (patients with a thrombophilia), have to use traneksamovy acid only on strict medical indications and under strict observation of the doctor. Use of traneksamovy acid in cases of the raised fibrinolysis owing to disseminate intravascular fibrillation is not recommended. Clinical experience of use of traneksamovy acid for children with a menorrhagia aged to 15 years is absent.
In a renal failure (depending on extent of increase in creatinine of serum) reduce a dose and frequency rate of introduction.
Before use of traneksamovy acid it is necessary to investigate risk factors of thromboembolic diseases. The patients having a disorder of vision have to stop treatment. The patients accepting oral contraceptives because of the increased risk of developing thrombosis should appoint Traneksamovy acid with care.
Pregnancy and the period of a lactation
of Traneksamovaya acid gets through a placenta. In researches on animals there are no proofs that traneksamovy acid has teratogenic effect, however pregnant women should use drug with care. Traneksamovy acid gets into breast milk in concentration about one cell from concentration in mother's blood. The anti-fibrinolytic effect at the baby is improbable. However it is necessary to use with care drug at the women nursing.
Features of influence of medicine on ability to run the vehicle and potentially dangerous mechanisms
Considering side effects of medicine it is necessary to be careful at control of motor transport and potentially dangerous mechanisms.
Overdose
Symptoms: nausea, vomiting or orthostatic hypotension.
Treatment: gastric lavage, activated carbon, plentiful drink, symptomatic, perhaps anticoagulating treatment.
The form of release and packing
On 6 tablets place in planimetric bezjyacheykovy packing from aluminum foil.
On 2 packs together with the instruction for medical use in the state and Russian languages put in a cardboard pack.
To Store storage conditions in the dry, protected from light place at a temperature not above 25 °C.
To store out of children's reach!
A period of storage
2 years
not to use after an expiration date
Prescription status
According to the prescription
the Name and the country
of the Macleods Pharmaceuticals Limited manufacturing organization, India
304, Atlanta Arcade, Marol Church Road, Andheri (East), Mumbai – 400,059, India.
The owner of the registration certificate
of Macleods Pharmaceuticals Limited, India
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products Branch KOO "Macleods Pharmaceuticals Limited" RK, Almaty, Tulebayev St. 38, office 307 Ph./fax. +7 727 2734593, 2441294, E-mail:
To Develop danielt@macleodspharma.com