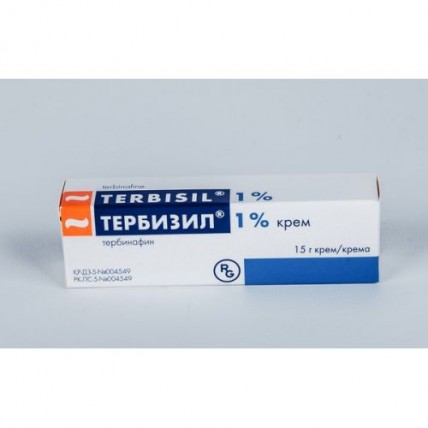
Terbizil 1% cream 15g
- $19.00
The instruction for medical use of TERBIZIL® medicine the Trade name of Terbizil® the International unlicensed name Terbinafin Lekarstvennaya a form Cream, 1% 15 g Structure 1 tuba contains active agent - terbinafin a hydrochloride of 0.150 g excipients: sodium hydroxide, benzyl alcohol, sorbitan monostearate, cetyl palmitate, cetyl alcohol, cetostearyl alcohol, polysorbate 60, isopropyl myristate, water purified sodium hydroxide of 2.4% solution the Description the Cream of white or almost white color with a light smell of almonds which is easily washed away by water Pharmacotherapeutic group Antifungal drugs for treatment of diseases of skin. Antifungal drugs for topical administration. Other antifungal drugs for topical administration. Terbinafin the ATX D01AE15 Code the Pharmacological Pharmacokinetics At properties topical administration is soaked up less than 5% of a dose therefore system influence is extremely small. After 7 days of use of the drug Terbizil® cream the kontsetration in a corneal layer of the processed area is higher than fungicidal and remains within at least 7 more days after the end of treatment. The pharmacodynamics of Terbizil® is the allylamine having a wide range of antifungal action. In small concentration drug has fungicide effect concerning dermatophytes, yeast-like mushrooms and some dimorphous mushrooms. The activity concerning barmy mushrooms, for example, of the sort Candida, can be fungicidal or fungistatic, depending on their look. Тербизил® suppresses an early stage of biosynthesis of sterols in a mushroom cell. It results in deficiency of ergosterol and intracellular accumulation of squalene and causes death of a cell of a mushroom. Terbinafin inhibits the enzyme to a skvalenepoksidaz located on a cellular membrane of a mushroom. Enzyme of a skvalenepoksidaz does not belong to the system of P450 cytochrome. Terbinafin does not influence metabolism of hormones or other medicines. Indications - the onychomycoses and mycoses of skin caused by dermatophytes: Trichophyton (for example, T. rubrum, T. mentagrophytes, T. verrucosum, T. violaceum), Microsporum canis and Epidermophyton floccosum: a foot epidermophitia (athlete's foot), a foot epidermophitia with defeat of a sole and its edges, an epidermophitia of inguinal area and smooth skin. - the skin infections caused by barmy mushrooms, the sorts Candida (for example, Candida Albicans). - the chromophytosis caused by Malassezia furfur the Route of administration and doses Terbizil® Cream is intended only for external use. Terbizil® cream can be used at adults and children 12 years are more senior. It is possible to use drug 1 or 2 times a day, depending on indications. Cream should be applied with a thin layer of 1 - 2 time a day on the affected skin and the surrounding area which is previously washed up and dried up slightly to rub. In the infections which are followed by an intertrigo (under a breast, between fingers, in interrump, in inguinal area) to the place of use it is possible to apply a gauze bandage, especially at night. Duration of treatment and frequency rate of use of drug Fungal infections of skin of a body, groin (trichophytia of smooth skin, an epidermophitia of inguinal area) - 1 week, 1 time a day Mycosis of feet (epidermophitia of feet) - 1 week, 1 time a day the foot Epidermophitia striking a sole and its edges - 2 weeks, 2 times a day skin Candidiasis - 1 week, 1 or 2 times a day the Chromophytosis - 2 weeks, 1 or 2 times a day Positive dynamics of clinical symptoms in a fungal infection comes within several days from the beginning of therapy. Irregular use and/or the premature termination of treatment can lead to a recurrence. If improvement does not occur within two weeks from an initiation of treatment, it is necessary and to consult with the attending physician. Use elderly people have no data assuming that for patients of advanced age other schemes of dosing are required or that they observe other side effects, in comparison with younger patients. Children Experience of use of a terbinafin for children are aged younger than 12 years is limited therefore use of drug at patients of this age group cannot be recommended. Side effects In sites of application of drug can arise an itching, peeling, pain, irritation, pigmentation disturbance, burning, erubescence, crusting, etc. These harmless symptoms should be distinguished from reaction of hypersensitivity which is followed by rash. Similar reactions are observed seldom, but at the same time drug phase-out is required. At hit in eyes can cause irritation. Exacerbation of the main fungal infection is in rare instances possible. Very often (≥1/10) - the irritation in the site of application Is frequent (≥1/100 to & lt, 1/10) - an itching in the site of application, burning sensation in the site of application, pain in the site of application, reddening in the site of application, hypersensitivity in the site of application, peeling of skin, an itching, pain, rash Infrequently (≥ 1/1000 - & lt, 1/100) - xeroderma, disturbance of integrity of skin, emergence of a crust on skin, pigmentation disturbance, erubescence, burning of skin, rash, pain Seldom (≥ 1/10000 & lt, 1/1000) - symptoms of irritation of a conjunctiva - contact dermatitis, eczema, aggravation of symptoms is Very rare (& lt, 1/10000) - bullous dermatitis, urticaria Frequency is unknown (it cannot be estimated on the basis of the available data) - hypersensitivity of the Contraindication - hypersensitivity to active ingredient or to any of excipients - children's age up to 12 years - the lactation period Medicinal interactions Medicinal interactions for a terbinafin in the form of cream are not known. The special instructions Terbizil® Cream it is intended only for external use. It is necessary to avoid hit of cream in eyes. At accidental hit of cream in their eyes it is necessary to wash with flowing water immediately. At emergence of allergic reaction it is necessary to wash away cream and to stop treatment. Cream contains cetostearyl alcohol and cetyl alcohol which can cause local skin reactions (for example, contact dermatitis). Pregnancy and the period of a lactation Clinical experience of use of a terbinafin for pregnant women is absent. In the researches conducted on animal the teratogenic properties of a terbinafin were not revealed. Use of a terbinafin during pregnancy is limited, use of external forms of drug during pregnancy is possible only according to strict indications. Terbinafin is allocated with breast milk. At topical administration it is possible to expect only insignificant system effect. Use of Terbizil® cream is not recommended during feeding by a breast. Also it is not necessary to allow the child to contact with the processed leather, including breast skin. There Is no feature of influence of medicine on ability to run the vehicle and potentially dangerous mechanisms data that Terbizil® cream affects ability to drive the car or to operate difficult mechanisms. Overdose Terbizil® Cream is intended only for external use. Accidental intake of two 15-gram tubes of cream (containing 300 mg of a terbinafin) is equivalent to intake of the active agent which is contained in one tablet of 250 mg Terbizila®. Symptoms: headaches, dizziness, nausea, epigastric pains. The symptoms similar to overdose by tablets, at accidental intake of cream inside. Treatment: gastric lavage, intake of activated carbon and if necessary symptomatic therapy the Form of release and packing of 15 g of cream in tubas aluminum with the twisting polyethylene cover of white color with the perforation punch. On 1 tuba together with the instruction for medical use in the state and Russian languages put in a cardboard box. To Store storage conditions at a temperature not above 30 °C. To store out of children's reach! Not to apply a period of storage of 5 years after an expiration date. Prescription status Without prescription the Name and the country of the JSC Gideon Richter manufacturing organization, Hungary the Name and the country of the owner of the registration certificate of JSC Gideon Richter, Hungary the Name and the country of the organization of the packer of JSC Gideon Richter, Hungary the Address of the organization accepting in the territory of the Republic of Kazakhstan a claim (offer) from consumers on quality of medicines responsible for post-registration observation of safety medicinal a sredstvapredstavitelstvo of JSC Gideon Richter in RK E-mail: info@richter.kz Phone number: 8-(7272)-58-26-22, 8-(7272)-58-26-23
To develop
To develop