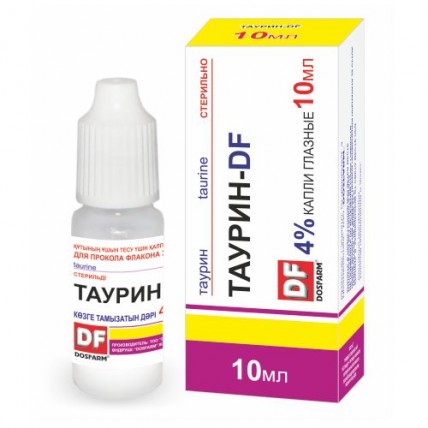
Taurine-DF Eye Drops 4%, 10 ml
- $4.00
Compound
1 ml of the drug contains
When applied topically, taurine penetrates into the environment of the eye, where it exerts its specific effect. When used in therapeutic doses, the drug is practically not absorbed into the systemic circulation.
Taurine-DF refers to amino acid drugs that stimulate reparative and regenerative processes in diseases of the retina of a dystrophic nature, traumatic lesions of eye tissues, pathological processes that are accompanied by a sharp violation of the metabolism of these tissues.
- injury and dystrophy of the cornea
- cataract (senile, diabetic, traumatic and radiation)
The drug is prescribed for adults.
Immediately before use, it is advisable to hold the vial with the drug in the palm of your hand to warm it up to body temperature.
In case of cataract Taurine-DF is prescribed as instillations 2-3 drops 2-4 times a day in the conjunctival sac of the affected eye for 3 months. Courses are repeated at monthly intervals.
For injuries and dystrophic diseases of the cornea, it is used in the same doses for 1 month.
It is necessary to ensure that when instilled, the tip of the dropper bottle does not come into contact with other objects or surfaces. After instillation, close the dropper bottle tightly with a cap!
Rarely:
- When using the drug, allergic reactions may occur in the form of allergic conjunctivitis caused by individual hypersensitivity to the components of the drug.
Visual disturbances:
- itchy eyes
- hyperemia of the conjunctiva
- eye irritation (cutting and burning in the eyes).
- hypersensitivity to the components of the drug
- children and adolescents up to 18 years of age
- pregnancy and lactation.
It is not recommended to use simultaneously with other eye drugs. If necessary, simultaneous use with other ophthalmic drugs, the interval between their use should be at least 15 minutes.
With simultaneous use with timolol eye drops, a potentiated decrease in intraocular pressure, as well as a potentiated decrease in blood pressure, is observed.
The medicinal product contains methyl parahydroxybenzoate, which may cause allergic reactions (possibly delayed type, bronchospasm).
Pregnancy and lactation
Clinical studies on the use of Taurine-DF in pregnant women and during lactation have not been conducted, so the drug is not prescribed to pregnant and lactating mothers.
Features of the influence of the drug on the ability to drive a vehicle or potentially dangerous mechanisms
Does not affect the ability to drive a vehicle or potentially dangerous machinery.
There are no data on drug overdose.
Store in a place protected from light at a temperature not exceeding 25°C.
Keep out of the reach of children!
Shelf life - 3 years
After opening the bottle, the period of application is 28 days.
Do not use after the expiration date.
1 ml of the drug contains
- Active ingredient - Taurine 0.04 g,
- Excipients: methyl parahydroxybenzoate, water for injection.
Pharmacological properties
Pharmacokinetics
When applied topically, taurine penetrates into the environment of the eye, where it exerts its specific effect. When used in therapeutic doses, the drug is practically not absorbed into the systemic circulation.
Pharmacodynamics
Taurine-DF refers to amino acid drugs that stimulate reparative and regenerative processes in diseases of the retina of a dystrophic nature, traumatic lesions of eye tissues, pathological processes that are accompanied by a sharp violation of the metabolism of these tissues.
Indications for use
- injury and dystrophy of the cornea
- cataract (senile, diabetic, traumatic and radiation)
Dosage and administration
The drug is prescribed for adults.
Immediately before use, it is advisable to hold the vial with the drug in the palm of your hand to warm it up to body temperature.
In case of cataract Taurine-DF is prescribed as instillations 2-3 drops 2-4 times a day in the conjunctival sac of the affected eye for 3 months. Courses are repeated at monthly intervals.
For injuries and dystrophic diseases of the cornea, it is used in the same doses for 1 month.
It is necessary to ensure that when instilled, the tip of the dropper bottle does not come into contact with other objects or surfaces. After instillation, close the dropper bottle tightly with a cap!
Side effects
Rarely:
- When using the drug, allergic reactions may occur in the form of allergic conjunctivitis caused by individual hypersensitivity to the components of the drug.
Visual disturbances:
- itchy eyes
- hyperemia of the conjunctiva
- eye irritation (cutting and burning in the eyes).
Contraindications
- hypersensitivity to the components of the drug
- children and adolescents up to 18 years of age
- pregnancy and lactation.
Drug Interactions
It is not recommended to use simultaneously with other eye drugs. If necessary, simultaneous use with other ophthalmic drugs, the interval between their use should be at least 15 minutes.
With simultaneous use with timolol eye drops, a potentiated decrease in intraocular pressure, as well as a potentiated decrease in blood pressure, is observed.
Special instructions
The medicinal product contains methyl parahydroxybenzoate, which may cause allergic reactions (possibly delayed type, bronchospasm).
Pregnancy and lactation
Clinical studies on the use of Taurine-DF in pregnant women and during lactation have not been conducted, so the drug is not prescribed to pregnant and lactating mothers.
Features of the influence of the drug on the ability to drive a vehicle or potentially dangerous mechanisms
Does not affect the ability to drive a vehicle or potentially dangerous machinery.
Overdose
There are no data on drug overdose.
Storage conditions
Store in a place protected from light at a temperature not exceeding 25°C.
Keep out of the reach of children!
Shelf life - 3 years
After opening the bottle, the period of application is 28 days.
Do not use after the expiration date.