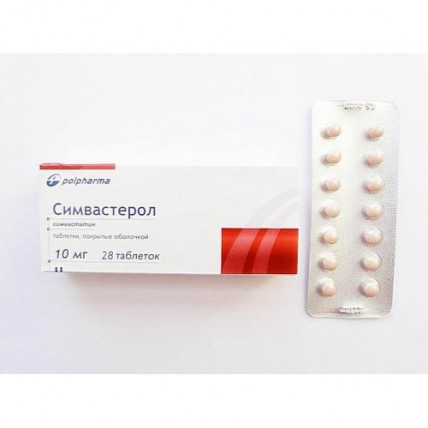
Simvasterol 28's 10 mg coated tablets
- $13.80
The instruction for use
of medicine for experts
of SIMVASTEROL
the Trade name
Simvasterol
Mezhdunarodnoye the unlicensed
name Simvastatin Simvastatin Lekarstvennaya
the Tablet form, coated 5 mg, 10 mg, 20 mg or 40 mg
Structure
One tablet contains
active agent - simvastatin 5 mg, 10 mg, 20 mg or 40 mg,
excipients: microcrystalline cellulose, krospovidon, magnesium stearate, butylhydroxyanisole (E 320), citric acid monohydrate (E 330), ascorbic acid (E 300),
structure of a cover: gipromelloza, lactose monohydrate, triacetate of glycerin, titanium dioxide (E 171), macrogoal 3000, oxides of iron (E 172).
The description
of the Tablet beige-pink (10 mg), pink (20 mg) or brown-pink color (40 mg), round, biconvex
Pharmacotherapeutic group
the Drugs reducing the maintenance of lipids in blood.
HMG-CoA reductase inhibitor.
ATC C code 10 AA01
Pharmacological
Pharmacokinetics Absorption properties of a simvastatin high. After oral administration the maximum concentration in blood plasma (Cmax) is reached in 1.3 - 2.4 h and in 12 h decreases by 90%. It is established that biological availability makes less than 5% of a dose. Linking with proteins of plasma makes 95%. It is metabolized in a liver, is exposed to effect of the first passing through a liver (it is generally hydrolyzed in the active form beta hydroxyacid, also other active and also inactive metabolites are found). Elimination half-life (T1/2) of active metabolites makes 1.9 h. It is generally removed through digestive tract (60%) in the form of metabolites. About 10 - 15% are removed by kidneys in an inactive form.
The pharmacodynamics
Simvastatin (synthetic derivative a product of fermentation Aspergillus terreus) is the pro-medicine reducing the maintenance of atherogenous lipids.
After intake, simvastatin, being an inactive lactone, it is hydrolyzed to the corresponding beta hydroxyacid which specifically reversibly inhibits 3-hydroxy-3-methylglutaryl coenzyme A (GMG-KoA) - reductase – the enzyme catalyzing transformation GMG-KoA in mevalonat. As a result of braking of this key link limiting the speed of biosynthesis of cholesterol at early stages simvastatin reduces the content of the general cholesterol, cholesterol of lipoproteids of the low density (LDL), lipoproteids of very low density (LPONP) and to a lesser extent triglycerides. Simvastatin moderately increases the level of cholesterol of lipoproteids of the high density (LPVP). Drug is highly effective at primary hypercholesterolemia when the dietotherapy is insufficient, in cases of heterozygous family and single forms of a hypercholesterolemia, the mixed lipidemia when the high level of cholesterol is risk factor. The expressed response to treatment develops within 2 weeks, and the maximum effect is reached within 4-6 weeks and remains throughout further treatment. Cancellation of medicine leads to restoration of level of cholesterol and lipids to initial, observed before treatment.
Indications
- coronary heart disease
At patients with coronary heart disease and concentration of the general cholesterol ≥ 5.5 mol/l (212 mg/dl) on purpose: decrease in the general mortality, reduction of risk of coronary mortality, reduction of risk of approach of not fatal myocardial infarction, decrease in probability of operational correction on restoration of a coronary blood-groove (aortocoronary shunting, chrezkozhny coronary angioplasty), delays of development of atherosclerotic process in coronary vessels, including delays of speed of development of new changes and a full obturation of a gleam of coronary vessels
- a hypercholesterolemia
Simvasterol is shown as the drug supplementing dietary treatment at patients with the increased concentration of the general cholesterol, LDL-cholesterol, apolipoprotein B and triglycerides during primary hypercholesterolemia, a family heterozygous hypercholesterolemia and also the mixed lipidemia when the diet and other ways of not pharmacological treatment are insufficient.
Use of the drug Simvasterol causes also increase in concentration of LPVP-cholesterol and by that reduction of the relation of LDL/LPVP and general cholesterol / LPVP.
A route of administration and doses
Before beginning to accept simvastatin, the patient has to pass to the standard diet reducing cholesterol level and to continue its observance during the Simvasterol drug treatment.
Coronary heart disease
At patients with coronary heart disease an initial dose makes 20 mg of 1 times a day, in the evening. In case of need it is possible to select a dose according to recommendations stated below (look: The hyper cholesteremia)
Giperkholisterinemiya
the Initial dose makes usually 10 mg of 1 times a day, in the evening, can be increased to the maximum daily dose of 40 mg. Usually therapeutic effect occurs in 2 weeks, the maximum effect is observed in 4 - 6 weeks of use. It is not necessary to increase a drug dose earlier, than in 4 weeks of treatment.
If the level of LDL cholesterol is lower than 75 mg/dl (1.94 mmol/l) or the level of the general cholesterol is lower than 140 mg/dl (3.6 mmol/l), it is necessary to reduce a drug Simvasterol dose.
Side effects
Simvastatin, as a rule, is well had.
The majority of side effects have gentle and tranzitorny disposition.
- seldom: a rhabdomyolysis, hepatitis or jaundice
- urticaria, hypersensitivity to light, fever, an erythema of the person, feeling of suffocation and feeling sick
- rash, an itching, baldness, myalgias
- weakness and headaches, dizzinesses, spasms, paresthesias, peripheral neuropathy
- abdominal pain, constipations, a meteorism, nausea, diarrhea, vomiting, pancreatitis
- arthritises and arthralgias, vasomotorial hypostasis, a pseudo-lupoid syndrome, rheumatic polymyalgia
- vasculites, thrombocytopenia, an eosinophilia, anemia, increase in the blood sedimentation rate (BSR)
- a myopathy which symptoms are myalgias, or muscle weakness and also substantial increase of activity of a creatine kinase (KK).
Contraindications
- hypersensitivity to any of drug components
- a liver disease in an active phase or permanent increase in level of content of transaminases in blood serum of the obscure etiology
- simultaneous use of a mibefradil
- a porphyria
- pregnancy and the period of a lactation
- children's age up to 18 years.
Medicinal interactions
Simvastatin is effective at monotherapy or in a combination with the medicines connecting bile acids.
The recommended maximum daily dose of a simvastatin at the persons accepting odnovremnenno cyclosporine, niacin or medicines from group of fibrat makes 10 mg.
Frequency of approach and intensity of the myopathy connected with use of GMG-KoA-reduktazy inhibitors is higher in case of simultaneous use with such medicines which can cause a myopathy, for example: gemfibrozit also other fibrata and also niacin (Niacinum) in the doses which are usually applied for the purpose of decrease in concentration of lipids (≥ 1 g/day). Simvastatin and other statines biotransformirutsya by isoenzyme. Some medicines, significantly oppressing in therapeutic doses 3A4 (CYP 3A4) cytochrome P - 450 zoenzy can cause substantial increase of concentration of GMG-KoA-reduktazy inhibitors in blood serum and thus increase risk of approach of a myopathy. Treat these medicines: cyclosporine, azolny antimycotic medicines (itrakonazol, ketokonazol and others), antibiotics macroleads (erythromycin and klaritromitsin), HIV virus protease inhibitors and also nefazodon (antidepressant).
It is necessary to resolve an issue of cancellation of a simvastatin at the patients accepting systematically antifungal antibiotics (itrakonazol, ketokonazol), or antibiotics from group of macroleads (erythromycin, klaritromitsin). It is not necessary to apply simvastatin along with other medicines which can oppress considerably in medical doses activity of CYP isoenzyme 3 A4, except cases when from the combined treatment the expected advantage is higher than potential risk.
Simvastatin coumarin derivatives in a daily dose from 20 to 40 mg are slightly strengthened by effect of anticoagulants from group of coumarin, the prothrombin time defined as International Normalized Ratio (INR) increased. At the patients undergoing treatment by anticoagulants from group of coumarin it is necessary to define INR before use of a simvastatin, a then to define it rather often in an initial stage of treatment to be convinced that there does not occur its lengthening.
simvastatin does not cause in the patients who are not undergoing treatment by anticoagulants from group of coumarin bleeding and changes of INR.
Digoxin
leads the Concomitant use of a simvastatin and digoxin to insignificant (less than 0.3 ng/ml) to increase in its concentration in blood plasma.
Other medicines
clinically negative interactions at simultaneous use of a simvastatin with inhibitors of angiotensin-convertin-enzyme (ACE), beta adrenolytic drugs, antagonists of calcium channels, diuretics and non-steroidal anti-inflammatory drugs are noted.
Special instructions
Ways of reduction of risk of the myopathy caused by medicinal interactions
before use of a simvastatin it is necessary to inform the patient on a possibility of development of a myopathy and to recommend to see immediately a doctor in case of appearance of the obscure muscular pains, their increased tactile sensitivity, or weakness. If, except above the listed symptoms, increase in activity of a creatine kinase (KK) in at least 10 times from the upper bound of norm is confirmed, it confirms the diagnosis of a myopathy. In that case it is necessary to cancel simvastatin.
It is necessary to watch carefully the patient to do not pass myopathy symptoms, especially in the first months of treatment and also after increase in a dose of drug. If there is no confidence that it is possible to provide with periodic determination of activity of a creatine kinase (KK) early diagnostics of a myopathy, it is necessary to resolve an issue of need of regular definition of KK.
Dosing in a renal failure
Simvastatin is not removed substantially by kidneys therefore persons with a moderate renal failure usually have no need for change of a drug dose.
At patients with a heavy renal failure (the clearance of creatinine
Influence on a liver
Is recommended to carry out an inspection of functional trials of a liver before an initiation of treatment, then periodically in the first year of use and for a year from the last increase in a drug dose. It is necessary to pay special attention to patients at whom increase in activity of transaminases is confirmed. In this group of persons of test it is necessary to repeat and increase the frequency of their control definition immediately. If there occurs further increase in activity of transaminases, especially if it keeps at the level of the values exceeding triple value of the upper bound of norm, medicine should be cancelled.
Medicine should be applied with care at the persons consuming significant amounts of alcohol or in liver diseases in the anamnesis. Liver diseases in an active phase or at superactivity of transaminases are contraindications to medicine use.
The control of vehicles and service of mechanisms
does not influence
Overdose
Symptoms: at use of very high doses of drug, collateral symptoms can amplify.
Treatment: in case of overdose it is necessary to cancel drug and to appoint symptomatic treatment.
Form of release and packing
of 5 mg, 10 mg, 20 mg: the packing containing 28 tablets in blisters from an Al/PVC foil in a cardboard pack with the instruction for use.
40 mg: the packing containing 14 tablets in blisters from an Al/PVC foil in a cardboard pack and also 28 tablets in blisters from an Al/PVC foil in a cardboard pack with the instruction for use.
To Store storage conditions in the dry, protected from light place, at a temperature below 25 °C.
To store out of children's reach!
An expiration date
2 years
not to use drug after expiry date.
Prescription status
According to the prescription
the Producer
the Pharmaceutical plant POLFARMA
S.A. St. of Pelplinsk 19, 83-200 Starogard Gdanski, Poland
to Develop
of medicine for experts
of SIMVASTEROL
the Trade name
Simvasterol
Mezhdunarodnoye the unlicensed
name Simvastatin Simvastatin Lekarstvennaya
the Tablet form, coated 5 mg, 10 mg, 20 mg or 40 mg
Structure
One tablet contains
active agent - simvastatin 5 mg, 10 mg, 20 mg or 40 mg,
excipients: microcrystalline cellulose, krospovidon, magnesium stearate, butylhydroxyanisole (E 320), citric acid monohydrate (E 330), ascorbic acid (E 300),
structure of a cover: gipromelloza, lactose monohydrate, triacetate of glycerin, titanium dioxide (E 171), macrogoal 3000, oxides of iron (E 172).
The description
of the Tablet beige-pink (10 mg), pink (20 mg) or brown-pink color (40 mg), round, biconvex
Pharmacotherapeutic group
the Drugs reducing the maintenance of lipids in blood.
HMG-CoA reductase inhibitor.
ATC C code 10 AA01
Pharmacological
Pharmacokinetics Absorption properties of a simvastatin high. After oral administration the maximum concentration in blood plasma (Cmax) is reached in 1.3 - 2.4 h and in 12 h decreases by 90%. It is established that biological availability makes less than 5% of a dose. Linking with proteins of plasma makes 95%. It is metabolized in a liver, is exposed to effect of the first passing through a liver (it is generally hydrolyzed in the active form beta hydroxyacid, also other active and also inactive metabolites are found). Elimination half-life (T1/2) of active metabolites makes 1.9 h. It is generally removed through digestive tract (60%) in the form of metabolites. About 10 - 15% are removed by kidneys in an inactive form.
The pharmacodynamics
Simvastatin (synthetic derivative a product of fermentation Aspergillus terreus) is the pro-medicine reducing the maintenance of atherogenous lipids.
After intake, simvastatin, being an inactive lactone, it is hydrolyzed to the corresponding beta hydroxyacid which specifically reversibly inhibits 3-hydroxy-3-methylglutaryl coenzyme A (GMG-KoA) - reductase – the enzyme catalyzing transformation GMG-KoA in mevalonat. As a result of braking of this key link limiting the speed of biosynthesis of cholesterol at early stages simvastatin reduces the content of the general cholesterol, cholesterol of lipoproteids of the low density (LDL), lipoproteids of very low density (LPONP) and to a lesser extent triglycerides. Simvastatin moderately increases the level of cholesterol of lipoproteids of the high density (LPVP). Drug is highly effective at primary hypercholesterolemia when the dietotherapy is insufficient, in cases of heterozygous family and single forms of a hypercholesterolemia, the mixed lipidemia when the high level of cholesterol is risk factor. The expressed response to treatment develops within 2 weeks, and the maximum effect is reached within 4-6 weeks and remains throughout further treatment. Cancellation of medicine leads to restoration of level of cholesterol and lipids to initial, observed before treatment.
Indications
- coronary heart disease
At patients with coronary heart disease and concentration of the general cholesterol ≥ 5.5 mol/l (212 mg/dl) on purpose: decrease in the general mortality, reduction of risk of coronary mortality, reduction of risk of approach of not fatal myocardial infarction, decrease in probability of operational correction on restoration of a coronary blood-groove (aortocoronary shunting, chrezkozhny coronary angioplasty), delays of development of atherosclerotic process in coronary vessels, including delays of speed of development of new changes and a full obturation of a gleam of coronary vessels
- a hypercholesterolemia
Simvasterol is shown as the drug supplementing dietary treatment at patients with the increased concentration of the general cholesterol, LDL-cholesterol, apolipoprotein B and triglycerides during primary hypercholesterolemia, a family heterozygous hypercholesterolemia and also the mixed lipidemia when the diet and other ways of not pharmacological treatment are insufficient.
Use of the drug Simvasterol causes also increase in concentration of LPVP-cholesterol and by that reduction of the relation of LDL/LPVP and general cholesterol / LPVP.
A route of administration and doses
Before beginning to accept simvastatin, the patient has to pass to the standard diet reducing cholesterol level and to continue its observance during the Simvasterol drug treatment.
Coronary heart disease
At patients with coronary heart disease an initial dose makes 20 mg of 1 times a day, in the evening. In case of need it is possible to select a dose according to recommendations stated below (look: The hyper cholesteremia)
Giperkholisterinemiya
the Initial dose makes usually 10 mg of 1 times a day, in the evening, can be increased to the maximum daily dose of 40 mg. Usually therapeutic effect occurs in 2 weeks, the maximum effect is observed in 4 - 6 weeks of use. It is not necessary to increase a drug dose earlier, than in 4 weeks of treatment.
If the level of LDL cholesterol is lower than 75 mg/dl (1.94 mmol/l) or the level of the general cholesterol is lower than 140 mg/dl (3.6 mmol/l), it is necessary to reduce a drug Simvasterol dose.
Side effects
Simvastatin, as a rule, is well had.
The majority of side effects have gentle and tranzitorny disposition.
- seldom: a rhabdomyolysis, hepatitis or jaundice
- urticaria, hypersensitivity to light, fever, an erythema of the person, feeling of suffocation and feeling sick
- rash, an itching, baldness, myalgias
- weakness and headaches, dizzinesses, spasms, paresthesias, peripheral neuropathy
- abdominal pain, constipations, a meteorism, nausea, diarrhea, vomiting, pancreatitis
- arthritises and arthralgias, vasomotorial hypostasis, a pseudo-lupoid syndrome, rheumatic polymyalgia
- vasculites, thrombocytopenia, an eosinophilia, anemia, increase in the blood sedimentation rate (BSR)
- a myopathy which symptoms are myalgias, or muscle weakness and also substantial increase of activity of a creatine kinase (KK).
Contraindications
- hypersensitivity to any of drug components
- a liver disease in an active phase or permanent increase in level of content of transaminases in blood serum of the obscure etiology
- simultaneous use of a mibefradil
- a porphyria
- pregnancy and the period of a lactation
- children's age up to 18 years.
Medicinal interactions
Simvastatin is effective at monotherapy or in a combination with the medicines connecting bile acids.
The recommended maximum daily dose of a simvastatin at the persons accepting odnovremnenno cyclosporine, niacin or medicines from group of fibrat makes 10 mg.
Frequency of approach and intensity of the myopathy connected with use of GMG-KoA-reduktazy inhibitors is higher in case of simultaneous use with such medicines which can cause a myopathy, for example: gemfibrozit also other fibrata and also niacin (Niacinum) in the doses which are usually applied for the purpose of decrease in concentration of lipids (≥ 1 g/day). Simvastatin and other statines biotransformirutsya by isoenzyme. Some medicines, significantly oppressing in therapeutic doses 3A4 (CYP 3A4) cytochrome P - 450 zoenzy can cause substantial increase of concentration of GMG-KoA-reduktazy inhibitors in blood serum and thus increase risk of approach of a myopathy. Treat these medicines: cyclosporine, azolny antimycotic medicines (itrakonazol, ketokonazol and others), antibiotics macroleads (erythromycin and klaritromitsin), HIV virus protease inhibitors and also nefazodon (antidepressant).
It is necessary to resolve an issue of cancellation of a simvastatin at the patients accepting systematically antifungal antibiotics (itrakonazol, ketokonazol), or antibiotics from group of macroleads (erythromycin, klaritromitsin). It is not necessary to apply simvastatin along with other medicines which can oppress considerably in medical doses activity of CYP isoenzyme 3 A4, except cases when from the combined treatment the expected advantage is higher than potential risk.
Simvastatin coumarin derivatives in a daily dose from 20 to 40 mg are slightly strengthened by effect of anticoagulants from group of coumarin, the prothrombin time defined as International Normalized Ratio (INR) increased. At the patients undergoing treatment by anticoagulants from group of coumarin it is necessary to define INR before use of a simvastatin, a then to define it rather often in an initial stage of treatment to be convinced that there does not occur its lengthening.
simvastatin does not cause in the patients who are not undergoing treatment by anticoagulants from group of coumarin bleeding and changes of INR.
Digoxin
leads the Concomitant use of a simvastatin and digoxin to insignificant (less than 0.3 ng/ml) to increase in its concentration in blood plasma.
Other medicines
clinically negative interactions at simultaneous use of a simvastatin with inhibitors of angiotensin-convertin-enzyme (ACE), beta adrenolytic drugs, antagonists of calcium channels, diuretics and non-steroidal anti-inflammatory drugs are noted.
Special instructions
Ways of reduction of risk of the myopathy caused by medicinal interactions
before use of a simvastatin it is necessary to inform the patient on a possibility of development of a myopathy and to recommend to see immediately a doctor in case of appearance of the obscure muscular pains, their increased tactile sensitivity, or weakness. If, except above the listed symptoms, increase in activity of a creatine kinase (KK) in at least 10 times from the upper bound of norm is confirmed, it confirms the diagnosis of a myopathy. In that case it is necessary to cancel simvastatin.
It is necessary to watch carefully the patient to do not pass myopathy symptoms, especially in the first months of treatment and also after increase in a dose of drug. If there is no confidence that it is possible to provide with periodic determination of activity of a creatine kinase (KK) early diagnostics of a myopathy, it is necessary to resolve an issue of need of regular definition of KK.
Dosing in a renal failure
Simvastatin is not removed substantially by kidneys therefore persons with a moderate renal failure usually have no need for change of a drug dose.
At patients with a heavy renal failure (the clearance of creatinine
Influence on a liver
Is recommended to carry out an inspection of functional trials of a liver before an initiation of treatment, then periodically in the first year of use and for a year from the last increase in a drug dose. It is necessary to pay special attention to patients at whom increase in activity of transaminases is confirmed. In this group of persons of test it is necessary to repeat and increase the frequency of their control definition immediately. If there occurs further increase in activity of transaminases, especially if it keeps at the level of the values exceeding triple value of the upper bound of norm, medicine should be cancelled.
Medicine should be applied with care at the persons consuming significant amounts of alcohol or in liver diseases in the anamnesis. Liver diseases in an active phase or at superactivity of transaminases are contraindications to medicine use.
The control of vehicles and service of mechanisms
does not influence
Overdose
Symptoms: at use of very high doses of drug, collateral symptoms can amplify.
Treatment: in case of overdose it is necessary to cancel drug and to appoint symptomatic treatment.
Form of release and packing
of 5 mg, 10 mg, 20 mg: the packing containing 28 tablets in blisters from an Al/PVC foil in a cardboard pack with the instruction for use.
40 mg: the packing containing 14 tablets in blisters from an Al/PVC foil in a cardboard pack and also 28 tablets in blisters from an Al/PVC foil in a cardboard pack with the instruction for use.
To Store storage conditions in the dry, protected from light place, at a temperature below 25 °C.
To store out of children's reach!
An expiration date
2 years
not to use drug after expiry date.
Prescription status
According to the prescription
the Producer
the Pharmaceutical plant POLFARMA
S.A. St. of Pelplinsk 19, 83-200 Starogard Gdanski, Poland
to Develop