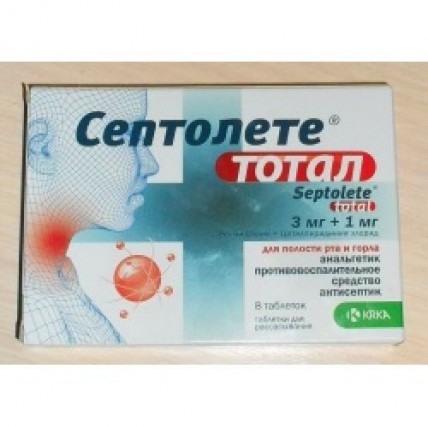
Septolete Total 8's lozenges
- $9.00
Out Of Stock
Sku:
8463390e33d1
The instruction for medical use of Septolete® medicine totat the Trade name of Septolete® totat the International unlicensed name there Is no Medicinal form of the Tablet for resorption Structure One tablet contains: active agents: benzydamine a hydrochloride of 3 mg, chloride cetylpyridinium monohydrate of 1.05 mg (it is equivalent cetylpyridinium to chloride of 1 mg), excipients: eucalyptus oil, left menthol, a sucralose (E955), citric acid anhydrous (E330), izomalt (type M) (E953), diamond blue FCF (E133) the Description of the Tablet of round shape, from blue till blue color, with a rough surface and a facet. Existence of unevenly painted, white spots and also existence of vials of air in the mass of lollipop and small gear edges Pharmacotherapeutic group Drugs for treatment of diseases of a throat is allowed. Antiseptic agents. Other drugs. The ATX R02AA20 code the Pharmacological Pharmacokinetics From properties of two active ingredients - cetylpyridinium chloride and benzydamine - is soaked up only benzydamine. Therefore cetylpyridinium chloride does not enter pharmacokinetic interaction with benzydamine at the system level. Absorption of benzydamine through mucous membranes of a mouth and throat was shown by means of identification of active ingredient in blood serum which number, nevertheless, was insufficiently for rendering systemic action. However, at system use, benzydamine is soaked up. Thus, the level of absorption of benzydamine is higher at use of dosage forms which are dissolved in an oral cavity, in comparison with dosage forms for topical administration (for example spray for an oral cavity). Distribution the Volume of distribution of all dosage forms is identical. Removal Drug is removed generally with urine, more, in the form of inactive metabolites. Elimination half-life and the general clearance are similar for all dosage forms. The pharmacodynamics the Mechanism of effect of Benzydamine a hydrochloride represents a molecule with nonsteroid chemical structure with the anti-inflammatory and anesthetizing properties. The action mechanism, is connected with inhibition of synthesis of prostaglandins and, thereby, with decrease in local signs of inflammation (such as pain, reddening, a swelling, burning). Benzydamine the hydrochloride also possesses moderate local anestetichesky action. Clinical performance and safety Benzydamine, is generally applied at treatment of diseases of a stomatopharynx. Cetylpyridinium chloride - an antiseptic agent from group of quarternary ammonium connections, has antimicrobial, antifungal and virulitsidny effect. Cetylpyridinium chloride is active concerning gram-positive bacteria and is less active concerning gram-negative bacteria, and, therefore, has antiseptic and bactericidal action. In placebo - controlled clinical trials of Septolete® totat tablets for resorption, the beginning of pain relief (decrease in painful feeling in a throat and a swelling in a throat) it was observed in 15 minutes after reception of a pastil and duration of action lasted till 3 o'clock. Indications the Adult as anti-inflammatory, anesthetic and antiseptic at irritation in a throat, an oral cavity and gums, in an ulitis, pharyngitis, laryngitis and tonsillitis, before exodontia. The route of administration and doses of the Tablet for resorption should be rassasyvat slowly in a mouth each 3 - 6 hours. Adult: the recommended dose makes 3-4 tablets for resorption of Septolete® totat in day. Tablets should be rassasyvat slowly in a mouth each 3 - 6 hours. To patients of advanced age: the recommended dose same, as well as for adult patients. It is contraindicated to children (cm. section Contraindications). For achievement of optimum effect, it is not recommended to use drug directly to or after toothbrushing. It is not necessary to exceed the recommended dose. Септолете® totat tablets for resorption it is possible to use within 7 days. Side effects Seldom (³1/10,000 to & lt, 1/1,000): hypersensitivity reactions bronchospasm small tortoiseshell, photosensitivity Very seldom (& lt, 1/10,000): the irritation of a mucous membrane of an oral cavity, burning sensation in an oral cavity Is unknown (it cannot be estimated on the basis of the available data): burning and/or numbness of a mucous membrane of an oral cavity of the Contraindication hypersensitivity to active agents or to any excipient the children's and teenage age up to 18 years Medicinal interactions of Septolete® totat tablets it is not necessary to apply to resorption along with other drugs from group of antiseptic agents. Pastils should not be accepted along with milk as milk chloride cetylpyridinium reduces antimicrobial efficiency. The special instructions Septolete® totat tablets it is not necessary to apply to resorption more than 7 days. In the absence of noticeable signs of improvement of a state, after 3 days of treatment it is necessary to consult the doctor. Use of local drugs, especially during the long span, can lead to reactions of a sensitization. In this case treatment has to be the corresponding therapy is suspended and is carried out. Септолете® totat tablets it is impossible to apply to resorption along with anion connections which are present at toothpastes therefore, it is not recommended to take the drug directly to or after toothbrushing. Totat tablets for resorption of Septolete® contain izomalt. Patients with rare hereditary problems of intolerance of fructose should not take this medicine. Pregnancy and the period of a lactation safety of use of benzydamine of a hydrochloride Given relatively and cetylpyridinium of chloride during pregnancy are absent or are limited. Септолете® totat tablets for resorption are not recommended for use during pregnancy. It is unknown whether the hydrochloride and its metabolites get into maternal milk of benzydamine. The risk for newborns/babies cannot be excluded. It is necessary to make the decision on the breastfeeding termination, or to refuse/refrain from therapy by the drug Septolete® totat, tablets for resorption, taking into account advantage of breastfeeding for the child and advantage of therapy for mother. Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms. About influence of Septolete® totat tablets for resorption on ability of control of vehicles and other mechanisms it was not reported. Overdose Symptoms: toxic manifestations of overdose of benzydamine include: excitement, spasms, the increased sweating, an ataxy, a fever and vomiting. In view of lack of specific antidote, treatment of acute intoxication benzydamine exclusively symptomatic. Signs and symptoms of intoxication at intake of significant amounts cetylpyridinium of chloride include: nausea, vomiting, short wind, cyanosis, asphyxia with the subsequent paralysis of respiratory muscles, oppression of central nervous system, arterial hypotension and a coma. Treatment: in view of lack of specific antidote, treatment of acute overdose exclusively symptomatic. Overdose treatment chloride cetylpyridinium also symptomatic. In case of overdose it is necessary to see a doctor Forma of release and packing On 8 tablets place in blister strip packaging from a film of polyvinylchloride/polyethylene/polyvinyldichloride and aluminum foil. On 1, 2 or 3 blister strip packagings together with the instruction for medical use in the state and Russian languages place in a pack from cardboard. To Store storage conditions in original packing at a temperature not over 30 ºС, in the place protected from light. To store out of children's reach! A period of storage 2 years not to apply after an expiration date Prescription status Without prescription the Producer of KRK, of. The place is new, Slovenia Shmaryeshka 6, 8501 the place, Slovenia the Owner of the registration certificate of KRK, of Is new. The place is new, Slovenia Shmaryeshka 6, 8501 the place, Slovenia Is new