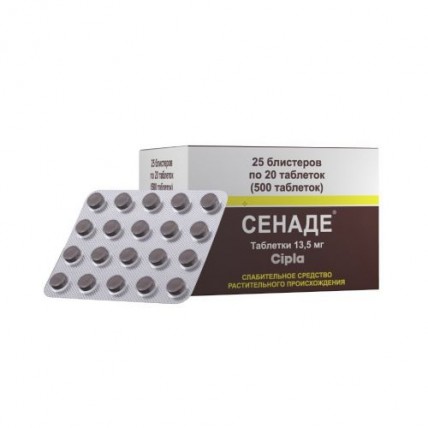
Senade 13.5 mg, 500 tablets
- $43.00
Out Of Stock
The instruction for medical use of medicine SENADUM the Trade name Senadum the International unlicensed name Is not present the Dosage form of the Tablet of 13.5 mg Structure One tablet contains active agent – calcium sennozid 15%..................... 90.00 mg (it is equivalent to 13.5 mg of Senna of calcic salts of sennozid And yes C), excipients: lactose, starch corn, cellulose microcrystalline, the talc purified, methylparahydroxybenzoate, magnesium stearate, sodium lauryl sulfate, sodium of a karmelloz. The description of the Tablet of round shape, with a flat surface with slanted edges, from light brown till dark brown color, with the central dividing line on one party and the squeezed-out monogram of CIPLA on other party. Pharmacotherapeutic group Digestive tract and metabolism. Laxatives. Contact laxatives. Sennas glycosides. ATH A06AB06 code the Pharmacological Pharmacokinetics properties β-Ο-svyazanny glycosides (sennozid) are not absorbed in an upper part of intestines and not split under the influence of human digestive enzymes. Under the influence of bacteria of a large intestine they turn into an active metabolite (reinovy Anteronum). Aglyca are absorbed in an upper part of intestines. At contact with oxygen reinovy Anteronum is oxidized to Rhine and sennidin which can be found in blood, generally in the form of glucuronides and sulfates. After oral administration of sennozid, 3 - 6% of metabolites are brought out of an organism with urine, a number is removed with bile. The majority of sennozid (about 90%) is removed with a stake in the form of polymers (polyquinones) together with 2 - 6% of sennozid in an invariable look, sennidinam, reinovy Anteronum and Rhine. Use of the powder of pods of Senna (20 mg of sennozid) accepted orally within 7 days was shown that in blood the maximum concentration 100 ng reina/ml is found. Accumulation of Rhine was not observed. Active metabolites, for example, Rhine, in small amounts get into breast milk. Rein in small degree gets through a placenta. A pharmacodynamics Derivative 1.8 dihydroxy anthracenes possess laxative action, i.e. soften a chair or dilute dense masses. There are two various mechanisms of action: 1. The stimulation of a vermicular movement of a large intestine leading to acceleration of colic transit. 2. Impact on secretory processes by two mechanisms at the same time – suppression of absorption of water and electrolytes (Na+, Cl-) in epithelial cells of a large intestine (anti-absorbing effect) and also increase in penetration through impenetrable partitions and stimulation of discharge of water and electrolytes into a gleam of a large intestine (enhancement effect of secretion) that leads to increase in concentration of liquid and electrolytes in a gleam of a large intestine. Defecation begins in 6-10 hours after reception of sennozid because some time for transportation in thick intestines and metabolism before active connection is necessary. Indications - chronic constipations - regulation of a chair in hemorrhoids, a proctitis, anal cracks - preparation for X-ray inspections. The route of administration and doses Accept inside usually 1 time a day in the evening before going to bed, Adults and children are more senior than 12 years: on 1 tablet on reception before going to bed, washing down with water or any drink. The maximum daily dose – 3 tablets. Children of 6-12 years: on ½ tablets before going to bed. Maximum daily dose 2 tablets. In the course of selection the same dose should be accepted within several days and to gradually increase it by ½ tablets. If after achievement of the maximum dose within 3 days the defecation does not happen, it is necessary to see a doctor. Side effects Following undesirable effects are classified according to the frequency and the class of a system of bodies (CSB). Frequency categories are defined according to the designations MedDRA (Medical dictionary of terms of regulatory activity): Very often (≥1/10), it is frequent (≥1/100 to & lt, 1/10), is not frequent (≥1/1000 to & lt, 1/100), is rare (≥1/10000 to & lt, 1/1000), Is very rare (& lt, 1/10000), It is unknown (it cannot be estimated on the available data). Often - hypersensitivity reactions (itching, the rash localized or a generalized dieback). Perhaps - an abdominal pain as gripes, spasms with a liquid chair, a meteorism, nausea and vomiting, in particular, at patients with the angry large intestine. However these symptoms were observed in connection with individual overdose. In such cases, it is necessary to suspend administration of drug or to lower its dose - long administration of drug can cause metabolic disturbances of balance of liquid and electrolytes that will lead ment of secondary dehydration, hypotension, especially at elderly people, an albuminuria, a hamaturia and uraemia. Moreover, long reception can cause pigmentation of a mucous membrane of intestines which usually decreases after suspension of administration of drug by the patient. Treatment can lead to coloring by urine metabolites in yellow or henna-red color depending on values rn. Seldom - excessive administration of drug leads to a hypopotassemia. Hypopotassemia etiology multifactorial: intestinal transport of electrolytes strengthens a hypopotassemia that leads to heart troubles and muscle weakness, in particular, at simultaneous use of cardiac glycosides, diuretics, to increase in plasma concentration of renin, a secondary aldosteronism and decrease in absorption. These changes are usually reversible after phase-out of depletive. The message about the suspected undesirable reactions Important is the message about the suspected undesirable reactions during the period after medicine registration. It allows to conduct continuous monitoring of balance of advantage/risk of medicine. Health workers are asked to report about any suspected undesirable reactions through the local national system of the message. Contraindications - hypersensitivity to active agent or any of excipients - intestinal impassability and a stenosis, an atony, appendicitis, inflammatory diseases of a large intestine (for example Crohn's disease, ulcer colitis) - an abdominal pain of unknown origin, heavy dehydration with water and electrolytic metabolic disturbances - to persons with hereditary intolerance of fructose, deficiency of Lapp-lactases enzyme, glucose galactose malabsorption - children's age up to 6 years. Long-term abuse of depletive can lead medicinal interactions to a hypopotassemia which can become aggravated at simultaneous use of cardiac glycosides and also there can be an interaction with the antiarrhytmic drugs, drugs causing reversion of a sinoatrial rate (for example, quinidine), and it means that extension of an interval of QT will be to be caused. Simultaneous use with the drugs causing a hypopotassemia (such as diuretics, adrenokortikosteroida and drugs of a root of a licorice) can strengthen disturbance of balance of electrolytes. This drug is not recommended to be used within two hours to or after intake of other medicines as sennozid can lower absorption and efficiency of effect of other drugs. Special instructions This drug needs to be used with care and to observe at patients who at the same time accept cardiac glycosides, antiarrhytmic means concerning which it is known that they cause extension of an interval of QT, diuretics, adrenokortikosteroida or drugs of a root of a licorice. As well as the patients suffering from fecal traffic jams and not diagnosed, acute or persistent gastrointestinal disorders, for example, an abdominal pain, nausea and vomiting should not take all depletive, this drug if the attending physician does not recommend differently as these symptoms can be signs of the potential or already present intestinal impassability (obstruction). If depletive needs to be accepted every day, then it is necessary to define the cause of constipations. Long-term intake of depletive needs to be carried out with care. If the stimulating depletive is accepted longer, than during the short span, then it can lead to dysfunction of intestines and dependence on depletive. This medicine needs to be applied only if the therapeutic effect cannot be reached by means of the change of a diet or depletive increasing the volume of intestinal contents. If this medicine is applied at the adults suffering from incontinence a calla, then it is necessary to change to a thicket a sheet (lining) for prevention of long contact of skin with a stake. This OTC medicine are not recommended for long-term use (longer than 1 week) as diarrhea, dehydration and an atony of intestines can develop. It is necessary to be careful at patients with a renal failure or a liver failure as disturbance of balance of electrolytes can develop. Excipients Lactose As tablets of this drug contain lactose, it cannot be applied at patients with rare hereditary intolerance of a galactose, deficiency of lactase, a glucose galactose sprue. Metilparagidroksibenzoat This drug contains methylparahydroxybenzoate. It can cause allergic reactions (perhaps slowed down type). Pregnancy messages about undesirable or harmful effects of drug on pregnancy and fetation at its use in the recommended doses did not arrive. However, taking into account the experimental data on risk of genotoxicity obtained for several antranoid, for example, of oemodin and an aloe oemodin, drug is not recommended to be used in the first trimester of pregnancy. Drug, after its appointment, needs to be applied very carefully in the second and third trimesters of pregnancy and only on a temporary basis in cases when change of lifestyle and a diet, or use of the drugs increasing intestines contents volume do not yield results. The lactation is not recommended to use drug during breastfeeding as there are insufficient data on penetration of its metabolites into breast milk. Small amounts of active metabolites get into breast milk. It was not reported about laxative action at the babies receiving chest feeding. Features of influence of medicine on ability to run vehicles or potentially dangerous mechanisms Drug does not affect ability to drive the car and to work with the equipment. However it is necessary to check individual reaction to drug. Overdose Symptoms: main symptoms of overdose / abuse: an abdominal pain and heavy diarrhea with the subsequent loss of liquid and electrolytes which amounts need to be restored. The special danger of diarrhea is that it can cause exhaustion of reserves of potassium in an organism which will lead to disturbances from heart and a muscular asthenia, in particular, at simultaneous use of cardiac glycosides, diuretics, adrenokortikosteroid or drugs of a root of a licorice. Treatment: Treatment has to be supporting with reception of large amounts of liquid. It is necessary to conduct monitoring of the content of electrolytes, especially potassium. The overdose owing to long-term intake of the medicines containing antranoida can lead to toxic hepatitis. The form of release and packing On 20 tablets place in blister strip packaging from a film polyvinylchloride/polyethylene / polyvinyldichloride (PVH/PE/PVDH) and aluminum foil. On 25 planimetric packs together with the instruction for medical use in the state and Russian languages place in a pack from cardboard. To Store storage conditions in the dry place, at a temperature not above 25 °C. To store out of children's reach! A period of storage 3 years not to apply after an expiration date Prescription status Without prescription Tsipla Ltd. Producer of Meditab Spl. Pvt. Ltd., 352, Kundaim Indl. Estate, Kundaim, Goa, 403,115, India Owner of the registration certificate Tsipla Ltd. Mumbai, India the Name, the address and a contact information (phone, the fax, e-mail) of the organization in the territory of the Republic of Kazakhstan, the accepting claim (offer) on quality of medicines from consumers and responsible for post-registration observation of safety of medicine: TOO Adalan Timiryazev St. 42, peahens. 23 offices 202, 050057 Almaty Ph. + 727 269 54 59, e-mail:
reg@adalan.kz
reg@adalan.kz