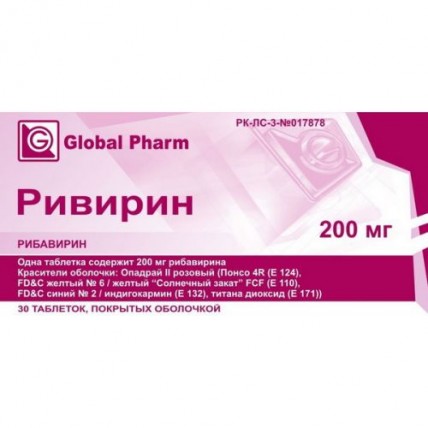
Rivirin 30s 200 mg coated tablets
- $46.20
Out Of Stock
The instruction for medical use
of Rivirin Torgovoye medicine a name
Rivirin
Mezhdunarodnoye the unlicensed
name Ribavirin Lekarstvennaya
the Tablet form, coated, 200 mg
Structure
One tablet contains
active agent – ribavirin 200 mg,
excipients: potato starch, sodium of starch glikolit, microcrystalline cellulose, magnesium or calcium stearate,
structure of a cover: Opadray II pink (Ponso 4R (E 124), FD&C Yellow No. 6/yellow Sunset of FCF (E 110), talc, FD&C Blue No. 2/indigo carmine (E 132), titan dioxide (E 171), polyvinyl alcohol, polyethyleneglycol / macrogoal).
The description
Round, biconvex tablets, coated from light pink till pink color, with risky on one party.
Pharmacotherapeutic group
Antiviral drugs of direct action. Nucleosides and nucleotides.
Code of automatic telephone exchange J05AB04
Pharmacological
Pharmacokinetics Absorption properties: at oral administration ribavirin it is quickly soaked up in digestive tract. At the same time its bioavailability is more than 45%.
Distribution: ribavirin it is distributed in plasma, secretion mucous airways and erythrocytes. A large number ribavirin triphosphate collects in erythrocytes, reaching constant concentration by 4th day and remaining within several weeks after introduction. The period of semi-distribution is 3.7 h. The volume of distribution (Vd) – 647-802 l. At course reception ribavirin collects in blood plasma in large numbers. The ratio of indicators of bioavailability (AUC – the area under a curve concentration time) at repeated and single dose is equal to 6. Considerable concentration of a ribavirin (more than 67%) can be found in cerebrospinal liquid after prolonged use. Slightly contacts proteins of blood plasma.
Time of achievement of the maximum concentration in blood plasma - from 1 to 1.5 hours.
Time of achievement of therapeutic concentration in blood plasma depends on the size of minute volume of blood.
The average size of the maximum concentration (Smakh) in blood plasma is about 5 - 11 µmol l at the end of 1 week of reception in a dose of 200 - 400 mg each 8 hours respectively.
Biotransformation: ribavirin it is phosphorylated in liver cells in active metabolites in the form of mono - di - and triphosphate which then metabilizirutsya in 1,2,4 – triazolkarboksamid (amide hydrolysis in trikarboksilovy acid and a deribozilirovaniye with formation of a triazolny carboxyl metabolite). Removal: ribavirin it is brought out of an organism slowly. Time of semi-removal (T1/2) after single dose of a dose of 200 mg is from 1 to 2 hours of plasma and up to 40 days from erythrocytes. After the termination of course reception of T1/2 Ribavirin and his metabolites makes about 300 h, are generally brought out of an organism with urine. Only 10% are removed with a stake. In an invariable look about 7% of a ribavirin are removed in 24 hours and about 10% - in 48 hours.
At administration of drug by patients with a renal failure of AUC and Smakh of a ribavirin increase that is caused by decrease in true clearance. At patients with a liver failure (And, In and From degree) the pharmacokinetics of a ribavirin does not change. After reception of a single dose with the food containing fats, the pharmacokinetics of a ribavirin significantly changes (AUC and Smakh increase by 70%). For the purpose of achievement of optimum concentration of a ribavirin in plasma the drug is recommended to be taken together with food.
The pharmacodynamics
Ribavirin, active agent of the drug Rivirin, is the synthetic nukleozidny analog active against RNA - and DNA viruses.
Quickly gets into cells and works in the cells infected with a virus. Intracellularly ribavirin it is easily phosphorylated by an adenosine kinasa to mono - di - and trifosfatny metabolites. Ribavirina triphosphate - a strong competitive inhibitor of inosine-monophosphate-dehydrogenase, a RNA polymerase of an influenza virus and guanilil-transferase of information RNA, the last is shown by slowing down of process of a covering information RNA cover. These various effects lead to considerable decrease in quantity intracellular triphosphate guanine riboside and also to suppression of synthesis of virus RNA and protein. Ribavirin inhibits replication of new virions that provides decrease in viral load, selectively inhibits synthesis of virus RNA, without suppressing synthesis of RNA in normally functioning cells.
It is most active concerning DNA viruses: Herpes simplex virus of types 1 and 2, adenoviruses, cytomegalovirus (CMV), virus of natural smallpox, Marek's disease. It is active concerning RNA viruses: influenza viruses And, In, paramyxoviruses (parainfluenza, respiratory syncytial virus, epidemic parotitis, the Newcastle disease), reoviruses, arenavirusa (virus of fever Lass, the Bolivian hemorrhagic fever), bunyavirusa (the Valley fever virus a rift, the Crimea-Congo virus of hemorrhagic fever, a hantavirusa which cause hemorrhagic fevers with a renal or pulmonic syndrome), oncogenous RNA viruses (human papillomavirus).
DNA viruses – chicken pox, a virus of AD, a natural vaccinia, RNA viruses - enteroviruses, rhinoviruses, a virus of encephalitis of the wood Semliki are insensitive to a ribavirin.
Rivirin has activity against a virus of hepatitis C (HCV). The triphosphate collecting in process of phosphorylation ribavirin competitively suppresses education triphosphate guanine riboside, thereby reducing synthesis of virus RNA. The mechanism of synergy action of a ribavirin and an alpha of interferon against VGS is caused by strengthening of phosphorylation of a ribavirin interferon.
As a part of combination therapy with interferon alpha 2a or 2b (standard or pegylated)
- chronic hepatitis C, including from the outcome
the doctor who has experience of treatment of patients with chronic hepatitis S.
Rivirin has to carry out indications to the compensated cirrhosis the Route of administration and doses Antiviral therapy (PVT) including interferon alpha 2a or 2b and ribavirin should not be applied as uniform therapeutic remedy as ribavirin it is inefficient as monotherapy of hepatitis C.
Use in a combination with peginterferon alpha 2a or 2b (пегИФНα-2а or 2b)
the Daily dose of Rivirin in a combination with пегИФНα-2а or 2b is determined by the patient's weight, and duration of treatment is calculated on the basis of hepatitis C virus genotype.
The daily dose of Rivirin is accepted daily inside in two steps in the morning and in the evening together with food.
The patients infected with a genotype 1 HCV at whom HCV RNA is defined on the 4th week irrespective of viral load have to receive treatment within 48 weeks.
24 weeks treatment is possible at patients with a genotype 1 in case of initial low viral load (NVN, no more than 800000 ME/ml) and the prompt virologic reply (PVR) on the 4th week and with lack of HCV RNA on the 24th week of treatment. However treatment lasting 24-weeks can be connected as a result with the increased risk of a recurrence, than 48 weeks treatment. Therefore when the issue of treatment duration is resolved, it is necessary to take such factors as shipping of combination therapy and also liver fibrosis degree into account. Shortening of a course of therapy at patients with a genotype 1 and initial high viral load (VVN, more than 800000 ME/ml) at which RNA of a virus is not defined on the 4th week and remains at not determined level on the 24th week, it is necessary to allow with bigger care as data of clinical trials do not allow to exclude negative impact of VVN on the steady virologic answer (table 1). In the presence of the slow virologic answer duration of PVT can be prolonged up to 72 weeks.
Patients with genotypes 2 or 3 viruses of hepatitis C at which HCV RNA is defined on the 4th week irrespective of initial viral load, can be limited to a 24 weeks course of treatment. Treatment lasting 16 weeks is possible at patients with a genotype 2 or 3 with the initial low viral load (LVL) at which RNA of a virus is not defined on the 4th week of treatment. However, at a 16 weeks course of treatment the risk of a recurrence is higher, than at a 24 weeks course of treatment. At patients of the given group at making decision on duration of therapy it is necessary to take shipping of the combined treatment and degree of fibrosis of a liver into consideration. Shortening of duration of treatment at patients with genotypes 2 or 3 with initial high viral load at which RNA of a virus is not defined on the 4th week, it is necessary to allow with bigger care as negative impact of VVN on the steady virologic answer is not excluded.
For patients with genotypes 5 or 6 the 48 weeks course of treatment пегИФНα-2а or 2b in a combination with Rivirin is recommended to a dose of 1000-1200 mg.
Table 1.
NVN, BVO
does not have the recommendation about dosing at the combined treatment of patients with a viral hepatitis S. Genotip of a virus pegifn pegifn Rivirin's Dose in day of Prodolzhitelnost of treatment α-2a α-2b Genotip 1, * 180 mkg 1.5 mkg/kg> 75 kg = 1200 mg of 24 or 48 weeks Genotip 1, VVN, BVO * 180 mkg 1.5 mkg/kg> 75 kg = 1200 mg of 48 weeks Genotip 4, BVI * 180 mkg 1.5 mkg/kg> 75 kg = 1200 mg of 24 or 48 weeks Genotip 1 or 4, BVI *
180
mkg 1.5 mkg/kg
> 75 kg = 1200 mg
of 48 weeks
Genotip 2 or 3, NVN, BVO *
180
mkg 1.5 mkg/kg 800 mg
16 or 24 weeks
Genotip 2 or 3, VVN, BVO *
180
mkg 1.5 mkg/kg 800 mg
24 weeks
Genotip 2 or 3, without BVI *
180
mkg 1.5 mkg/kg 800 mg
24 weeks
of BVI * - prompt virologic reply (RNA of a virus is not defined on the 4th week).
NVN – low viral load (no more than 800000ME/ml),
VVN – high viral load (more than 800000 ME/ml).
Table 2. Calculation of a daily dose of Rivirin.
The body weight (kg)
Rivirin
the Daily dose
(mg)
Quantity
of tablets 40 - 65 kg
800 2 tablets in the morning +2 tablets in the evening
of 66-80 kg
1000 2 tablets in the morning +3 tablets in the evening
81-105kg 1200 3 tablets in the morning +3 tablets in the evening
>
105 1400 3 tablets in the morning +4 tablets in the evening
Table 3. Recommendations about change of a dosage of Rivirin for elimination of the anemia which arose as a result of treatment.
The laboratory
Reduce a Dose by 200 Mg/days values * and/or it is necessary to appoint erythropoietin only if:
Administration of drug should be stopped if **:
Hemoglobin
of 85 g/l
Hemoglobin at patients with heart diseases
decrease more, than on 2 g/dl within any 4 weeks during therapy (continuous reduction of a dose)
* patients to whom Rivirin's dose decreases to 600 mg a day accept on one 200-mg tablet in the morning and two tablets in the evening
** at normalization of indicators it is possible to start over again treatment by Rivirin in a dose of 600 mg a day, further increase in a dose up to 800 mg a day – to the discretion of the doctor. Return to higher doses is not recommended.
Chronic hepatitis C at inefficiency of the previous treatment
the Recommended Rivirin's dosage in a combination with пегИФНα-2а makes a day 1000 mg for patients with body weight less than 75 kg and 1200 mg for patients with the body weight of 75 kg and above, irrespective of a genotype, in a combination with пегИФНα-2b the daily dose makes 15мг/кг/сут. The recommended treatment duration for a genotype 1 or 4 is 72 weeks, and 48 weeks – for genotypes 2 or 3.
Use in a combination with standard interferon alfa-2
the Recommended Rivirin's doses at use in a combination with interferon alfa-2 depend on the body weight of the patient.
The body weight of the patient
the Daily dose
of Riverin Prodolzhitelnost of treatment
Quantity of tablets on 200 mg
<>
1000 mg
of 24 or 48 weeks
5 (in the 2nd morning and in the 3rd evening)
> 75
1200 mg
of 24 or 48 weeks
6 (in the 3rd morning and in the 3rd evening)
Prodolzhitelnost of treatment makes not less than 6 months. Patients with a genotype of virus 1 have to receive combination therapy within 48 weeks. For patients with other genotypes of a virus the decision on extension of therapy to 48 weeks has to be based on other predictive factors (such as initially high viral load, a male, age is more senior than 40 years and presence of bridge-like fibrosis of a liver at a histologic research of the bioptat).
Use at the compensated cirrhosis
to Patients with quantity of neutrophils more than 1.5·109/l and thrombocytes > 70·109/l peginterferon and Rivirin are appointed in a standard dose with weekly control of the general blood test.
At emergence of side effects or changes of laboratory indicators during treatment by Rivirin in a combination with пегИФНα-2 or interferon alfa-2 it is necessary to change doses of each of drugs before stopping of side reactions. At preservation of these phenomena after change of a dose of Rivirin, it is necessary to cancel treatment.
At lack of decrease in the HCV RNA level from a reference value on 2 log10 or more (≥100 times) in 12 weeks of treatment PVT should be stopped.
Side effects
Type and frequency of side effects as a result of combination therapy are caused by the known profile of safety of interferon alpha 2а or 2b or пегИФНα-2а or 2b and a ribavirina.
Often
- insomnia, irritability, a depression, loss of concentration of attention, a headache, dizziness
- decrease in body weight
- short wind, cough
- anorexia, nausea, diarrhea, an abdominal pain
- an alopecia, an itching, dermatitis, xeroderma
- myalgia, an arthralgia
- weakness, temperature increase, a fever, an asthenia
Infrequently
- herpes, infections of an urinary path, bronchitis, candidiasis of an oral cavity
- a lymphadenopathy
- anemia, thrombocytopenia
- a hypothyroidism, a hyperthyroidism
- disturbance of memory, paresthesia, a giposteziya, a tremor, weakness, dizziness, emotional lability, nervousness, aggression, decrease a libido, migraine, a hyperesthesia, nightmares, uneasiness, a syncope
- a zatumanennost in eyes, a xerophthalmia, conjunctivitis, eye pain
- ear pain
- heartbeat, tachycardia, inflows, an asthma at physical activity
- rhinitis, nasopharyngites, congestion of a nose, nasal bleeding
- dryness in a mouth, ulcerations of a mucous oral cavity, an odontorrhagia, stomatitis, a dysphagy, a glossitis, disturbance of taste, thirst, vomiting, dyspepsia, a meteorism
- rash, eczema, psoriasis, reactions of photosensitivity
- the increased perspiration, night sweats, peripheral hypostases
- an ostealgia, a stethalgia, in a back and a neck, muscle weakness, spasms of muscles, muscle pain, arthritis
- erectile dysfunction
- a grippopodobny symptom, fatigue
Seldom
- milk acidosis (hyper lactacidemia), the acquired lipodystrophy and a chromaturia
- flu, pneumonia, throat and throat pain, a cheilitis
- affective lability, apathy
- a ring in ears
Very seldom (isolated cases)
- lower respiratory tract infections, skin infections, external otitis
- suicide mood, psychotic disorders, hallucinations, peripheral neuropathy
- an abnormal liver function, fat dystrophy of a liver, a cholangitis, a malignant tumor of a liver
- a peptic ulcer of a stomach, gastrointestinal bleedings, pancreatitis
- arrhythmia, atrial fibrillation, a lowering of arterial pressure, a pericarditis, an endocarditis
- a coma and hemorrhage in a brain
- an embolism of a pulmonary artery
- autoimmune diseases (for example, a thyroiditis, myocarditis, a pseudorheumatism), a miositis, a sarcoidosis, an interstitial pneumonitis with a lethal outcome, a keratohelcosis
- a pancytopenia or aplastic anemia
the Side reactions connected with reception of a ribavirin
- decrease in level of hemoglobin, quantity of erythrocytes
-
Contraindication insomnia
- hypersensitivity to a ribavirin or any component of drug
- a serious illness of heart, including unstable and uncontrollable forms which are observed, at least, for 6 months prior to treatment
- pregnancy and the period of a lactation (therapy should not be begun data on negative take of the test for pregnancy)
- children's and teenage age up to 18 years
- hemoglobinopathies (for example, a thalassemia, a sickemia will not be obtained yet)
- a serious, wearisome illness, including at patients with chronic kidney disease or with clearance of creatinine is lower than 50 ml/min.
- a heavy abnormal liver function or dekompensirovanny cirrhosis
- autoimmune hepatitis or other autoimmune diseases in the anamnesis
- the profound depression and suicide moods
Medicinal interactions
Enzymes of P450 cytochrome do not participate in metabolic transformations of a ribavirin. Therefore there is the minimum probability for interaction with P450 cytochrome.
Antacids. The bioavailability of a ribavirin in a dose of 600 mg decreases at joint intake of the antiacid drug containing compounds of magnesium and aluminum or simetikon.
Analogs of nucleosides. Ribavirin oppresses phosphorylation of a zidovudine and stavudin. Rivirin's use with a zidovudine or stavudiny can lead to increase in concentration of HIV in blood plasma. Therefore careful monitoring of levels of concentration of RNK-VICh in blood plasma at patients who receive treatment by Rivirin in a combination with one of these two means is recommended. At increase in level of concentration of RNK-VICh in plasma, Rivirin's use with inhibitors of reverse transcriptase should be reconsidered.
Use of nukleozidny analogs separately or in a combination with other nucleosides, can lead to development of a lactacidemia. Rivirin increases the maintenance of fosforilirovanny metabolites of purine nucleosides. This effect can exponentiate risk of developing of the lactacidemia caused by purine analogs of nucleosides (for example didanozin or abakavir).
At the HIV-positive patients receiving VAART (highly active antiretroviral therapy) the risk of developing of lactoacidosis increases. Therefore it is necessary to perform carefully combination therapy against the background of VAART.
The possibility of interaction with Rivirin remains for two months (5 elimination half-life of a ribavirin) after phase-out, thanks to long elimination half-life.
The special
instructions Use of a Ribavirin as monotherapy inefficiently therefore Rivirin should not be applied independently at treatment of hepatitis C. Evening administration of drug no later than 19:00 hours as one of side effects is insomnia is recommended.
Hemolysis/anemia
Though drug has no direct impact on a cardiovascular system, the anemia connected with Rivirin's reception can strengthen heart failure and/or provoke aggravation of symptoms of a coronary disease. Therefore therapy by Rivirin in a combination with peginterferon alpha 2а or 2b or interferon alpha 2а or 2b, has to be appointed with care to patients with heart diseases. It is necessary to estimate a condition of a cardiovascular system before an initiation of treatment and during therapy to carry out clinical monitoring, in case of identification of any signs of deterioration from a cardiovascular system the therapy has to be stopped.
Owing to hemolysis the level of uric acid in blood serum can increase. Therefore, considering, risk of developing gout needs to be watched the patients predisposed to this disease carefully.
A cardiovascular system
Adult patients who have signs (or in the anamnesis) stagnant heart failure, a myocardial infarction and/or arrhythmia, have to be under constant observation of the doctor. At patients with heart diseases before the beginning and during treatment it is recommended to carry out electrocardiography. Arrhythmias (generally supraventricular), as a rule, give in to traditional therapy, but can demand the therapy termination.
Immediate hypersensitivity
At development of acute reaction of hypersensitivity (for example, small tortoiseshells, a Quincke's disease, a bronchospasm, an anaphylaxis) Rivirin's use it is necessary to stop and appoint the corresponding treatment immediately. Tranzitorny rashes are not the basis for the treatment termination.
Use in an abnormal liver function
of Pharmacokinetic interaction between ribaviriny and function of a liver is not revealed. Therefore for patients with a liver failure the modification of a dose of Rivirin in a combination with peginterferon alpha 2а or 2b or interferon alpha 2а or 2b is not required. Therapy should be stopped in case of progressing of signs and symptoms of an abnormal liver function. Rivirin in a combination with peginterferon alpha 2а or 2b or interferon alpha 2а or 2b is contraindicated in heavy dysfunction of a liver or in a dekompensirovanny disease of a liver.
Hepatotoxicity, including fatal, was observed seldom at use of interferon alpha 2а or 2b. At use of a ribavirin in a combination with interferon alpha 2а or 2b for the patient it is necessary to establish careful observation and control of laboratory indicators. If ALT (alaninaminotranspherase) is doubled up to the size or more exceeding a reference value, treatment can be continued in the absence of symptoms of a liver failure. At the same time, definition of ALT, nuclear heating plant (aspartate aminotransferase), a prothrombin time, alkaline phosphatase, albumine and bilirubin should be carried out every 2 week.
Use in a renal failure
Due to the considerable decrease in clearance of creatinine at patients with renal dysfunction, Rivirin's pharmacokinetics at such patients is broken. Therefore it is recommended to estimate function of kidneys at all patients prior to therapy by Rivirin. Patients with clearance of creatinine should not apply lower than 50 ml/min. Rivirin.
Use for elderly people (³ 65 years)
Is recommended to estimate function of kidneys prior to therapy.
Mental disturbances and the central nervous system
If it is decided that combination therapy with Rivirin is necessary for patients with the clinical or anamnestic data confirming heavy mental disturbance it should be begun only after performing the corresponding individual diagnostics and against the background of therapeutic maintaining a mental state. At development of heavy neuropsychiatric effects, especially depressions, Rivirin's use in a combination with peginterferon alpha 2а or 2b or interferon alpha 2а or 2b it is necessary to interrupt.
At emergence of mental disturbances or changes from central nervous system including clinic of a depression it is recommended to watch patients constantly during treatment and during further observation considering potential gravity of the similar undesirable phenomena. At preservation or increase of symptoms, or at identification of thoughts of a suicide or the agressive behavior directed on people around it is recommended to stop treatment and to render to the patient the appropriate mental health services.
Use at to - infection of HIV and hepatitis C virus
At the patients receiving therapy by nenukleozidny inhibitors of reverse transcriptase in a complex with a combination of a ribavirin and peginterferon alpha 2а or 2b/interferon alpha 2а or 2b can increase risk of development of mitochondrial toxicity, lactoacidosis and liver failure.
At to - the infected patients with cirrhosis receiving highly active antiretroviral therapy (VAART) risk of development of a hepatic decompensation and death increases. Additional appointment the alpha of interferon separately or in a combination with ribaviriny increases the above-stated risks at this category of patients.
Treatment of patients with the low level of CD of 4+ cells needs to be carried out carefully.
Dental and periodontal disturbances
the Dryness in a mouth can render the damaging effect on teeth and a mucous membrane of a mouth during long combination therapy by Rivirin, interferon alpha 2а or 2b or peginterferon alpha 2а or 2b. Patients should recommend to brush carefully teeth 2 times a day and to regularly undergo dental inspection. Besides, some patients can have a vomiting then they have to rinse an oral cavity carefully.
Laboratory researches.
All patients prior to therapy and during treatment (on the 2nd and 4th week and then as necessary) are recommended to carry out the general blood test (the developed analysis with definition of a leukocytic formula) and biochemical analysis of blood (electrolytes, serumal creatinine, hepatic tests, uric acid), a research of function of a thyroid gland.
The women of childbearing age
of the Woman receiving treatment, and women – sexual partners receiving treatment, have to monthly throughout the entire period of treatment and 6 months after completion of treatment to carry out tests for pregnancy.
Control of function of a thyroid gland
before PVT needs to be defined the level of thyroid stimulating hormone (TSH) and existence of antibodies to thyreoglobulin (TG) and thyroid peroxidase (TPO). Any deviations of thyroid function found at this time have to be modified by traditional therapy. If the content of TSH manages to be supported by medicamentous therapy at the normal level, antiviral treatment can be begun. At emergence of symptoms of dysfunction of a thyroid gland against the background of treatment it is necessary to determine by alpha interferon the thyroid status and to carry out the corresponding treatment.
Embriotoksichesky and teratogenic action
Ribavirin collects intracellularly and is brought out of an organism very slowly, therefore, can collect in sperm and show inherent in it teratogenic or genotoksichesky actions on a human embryo / fruit.
The feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
during treatment to the persons feeling fatigue, drowsiness or a disorientation needs to abstain from driving of motor transport and occupations potentially dangerous types of activity demanding the increased concentration of attention and speed of psychomotor reactions.
Overdose
Symptoms: strengthening of expressiveness of side effect is possible.
Treatment: drug withdrawal, symptomatic therapy.
A form of release and packing
On 10 tablets in blister strip packaging from a film of polyvinylchloride and aluminum foil.
On the 3rd blister strip packagings together with the instruction for medical use in the state and Russian languages place in a pack from cardboard.
To Store storage conditions in the dry, protected from light place at a temperature not over 250C.
To store out of children's reach!
A period of storage
2 years
not to use drug after the expiry date specified on packing.
Prescription status
According to the prescription
JV Global Pharm LLP Producer,
Republic of Kazakhstan. Almaty, Dzhandosov St. 184 of , ph. 309-74-07, 309-74-14.
The address of the organization of the claim accepting in the territory of the Republic of Kazakhstan from the consumer on quality of products:
JV Global Pharm LLP
050042, Republic of Kazakhstan, Almaty, Dzhandosov St. 184 of
ph. 309-74 - 07, fax 309-74-14. e-mail: globalzavod@mail.ru.
To develop
of Rivirin Torgovoye medicine a name
Rivirin
Mezhdunarodnoye the unlicensed
name Ribavirin Lekarstvennaya
the Tablet form, coated, 200 mg
Structure
One tablet contains
active agent – ribavirin 200 mg,
excipients: potato starch, sodium of starch glikolit, microcrystalline cellulose, magnesium or calcium stearate,
structure of a cover: Opadray II pink (Ponso 4R (E 124), FD&C Yellow No. 6/yellow Sunset of FCF (E 110), talc, FD&C Blue No. 2/indigo carmine (E 132), titan dioxide (E 171), polyvinyl alcohol, polyethyleneglycol / macrogoal).
The description
Round, biconvex tablets, coated from light pink till pink color, with risky on one party.
Pharmacotherapeutic group
Antiviral drugs of direct action. Nucleosides and nucleotides.
Code of automatic telephone exchange J05AB04
Pharmacological
Pharmacokinetics Absorption properties: at oral administration ribavirin it is quickly soaked up in digestive tract. At the same time its bioavailability is more than 45%.
Distribution: ribavirin it is distributed in plasma, secretion mucous airways and erythrocytes. A large number ribavirin triphosphate collects in erythrocytes, reaching constant concentration by 4th day and remaining within several weeks after introduction. The period of semi-distribution is 3.7 h. The volume of distribution (Vd) – 647-802 l. At course reception ribavirin collects in blood plasma in large numbers. The ratio of indicators of bioavailability (AUC – the area under a curve concentration time) at repeated and single dose is equal to 6. Considerable concentration of a ribavirin (more than 67%) can be found in cerebrospinal liquid after prolonged use. Slightly contacts proteins of blood plasma.
Time of achievement of the maximum concentration in blood plasma - from 1 to 1.5 hours.
Time of achievement of therapeutic concentration in blood plasma depends on the size of minute volume of blood.
The average size of the maximum concentration (Smakh) in blood plasma is about 5 - 11 µmol l at the end of 1 week of reception in a dose of 200 - 400 mg each 8 hours respectively.
Biotransformation: ribavirin it is phosphorylated in liver cells in active metabolites in the form of mono - di - and triphosphate which then metabilizirutsya in 1,2,4 – triazolkarboksamid (amide hydrolysis in trikarboksilovy acid and a deribozilirovaniye with formation of a triazolny carboxyl metabolite). Removal: ribavirin it is brought out of an organism slowly. Time of semi-removal (T1/2) after single dose of a dose of 200 mg is from 1 to 2 hours of plasma and up to 40 days from erythrocytes. After the termination of course reception of T1/2 Ribavirin and his metabolites makes about 300 h, are generally brought out of an organism with urine. Only 10% are removed with a stake. In an invariable look about 7% of a ribavirin are removed in 24 hours and about 10% - in 48 hours.
At administration of drug by patients with a renal failure of AUC and Smakh of a ribavirin increase that is caused by decrease in true clearance. At patients with a liver failure (And, In and From degree) the pharmacokinetics of a ribavirin does not change. After reception of a single dose with the food containing fats, the pharmacokinetics of a ribavirin significantly changes (AUC and Smakh increase by 70%). For the purpose of achievement of optimum concentration of a ribavirin in plasma the drug is recommended to be taken together with food.
The pharmacodynamics
Ribavirin, active agent of the drug Rivirin, is the synthetic nukleozidny analog active against RNA - and DNA viruses.
Quickly gets into cells and works in the cells infected with a virus. Intracellularly ribavirin it is easily phosphorylated by an adenosine kinasa to mono - di - and trifosfatny metabolites. Ribavirina triphosphate - a strong competitive inhibitor of inosine-monophosphate-dehydrogenase, a RNA polymerase of an influenza virus and guanilil-transferase of information RNA, the last is shown by slowing down of process of a covering information RNA cover. These various effects lead to considerable decrease in quantity intracellular triphosphate guanine riboside and also to suppression of synthesis of virus RNA and protein. Ribavirin inhibits replication of new virions that provides decrease in viral load, selectively inhibits synthesis of virus RNA, without suppressing synthesis of RNA in normally functioning cells.
It is most active concerning DNA viruses: Herpes simplex virus of types 1 and 2, adenoviruses, cytomegalovirus (CMV), virus of natural smallpox, Marek's disease. It is active concerning RNA viruses: influenza viruses And, In, paramyxoviruses (parainfluenza, respiratory syncytial virus, epidemic parotitis, the Newcastle disease), reoviruses, arenavirusa (virus of fever Lass, the Bolivian hemorrhagic fever), bunyavirusa (the Valley fever virus a rift, the Crimea-Congo virus of hemorrhagic fever, a hantavirusa which cause hemorrhagic fevers with a renal or pulmonic syndrome), oncogenous RNA viruses (human papillomavirus).
DNA viruses – chicken pox, a virus of AD, a natural vaccinia, RNA viruses - enteroviruses, rhinoviruses, a virus of encephalitis of the wood Semliki are insensitive to a ribavirin.
Rivirin has activity against a virus of hepatitis C (HCV). The triphosphate collecting in process of phosphorylation ribavirin competitively suppresses education triphosphate guanine riboside, thereby reducing synthesis of virus RNA. The mechanism of synergy action of a ribavirin and an alpha of interferon against VGS is caused by strengthening of phosphorylation of a ribavirin interferon.
As a part of combination therapy with interferon alpha 2a or 2b (standard or pegylated)
- chronic hepatitis C, including from the outcome
the doctor who has experience of treatment of patients with chronic hepatitis S.
Rivirin has to carry out indications to the compensated cirrhosis the Route of administration and doses Antiviral therapy (PVT) including interferon alpha 2a or 2b and ribavirin should not be applied as uniform therapeutic remedy as ribavirin it is inefficient as monotherapy of hepatitis C.
Use in a combination with peginterferon alpha 2a or 2b (пегИФНα-2а or 2b)
the Daily dose of Rivirin in a combination with пегИФНα-2а or 2b is determined by the patient's weight, and duration of treatment is calculated on the basis of hepatitis C virus genotype.
The daily dose of Rivirin is accepted daily inside in two steps in the morning and in the evening together with food.
The patients infected with a genotype 1 HCV at whom HCV RNA is defined on the 4th week irrespective of viral load have to receive treatment within 48 weeks.
24 weeks treatment is possible at patients with a genotype 1 in case of initial low viral load (NVN, no more than 800000 ME/ml) and the prompt virologic reply (PVR) on the 4th week and with lack of HCV RNA on the 24th week of treatment. However treatment lasting 24-weeks can be connected as a result with the increased risk of a recurrence, than 48 weeks treatment. Therefore when the issue of treatment duration is resolved, it is necessary to take such factors as shipping of combination therapy and also liver fibrosis degree into account. Shortening of a course of therapy at patients with a genotype 1 and initial high viral load (VVN, more than 800000 ME/ml) at which RNA of a virus is not defined on the 4th week and remains at not determined level on the 24th week, it is necessary to allow with bigger care as data of clinical trials do not allow to exclude negative impact of VVN on the steady virologic answer (table 1). In the presence of the slow virologic answer duration of PVT can be prolonged up to 72 weeks.
Patients with genotypes 2 or 3 viruses of hepatitis C at which HCV RNA is defined on the 4th week irrespective of initial viral load, can be limited to a 24 weeks course of treatment. Treatment lasting 16 weeks is possible at patients with a genotype 2 or 3 with the initial low viral load (LVL) at which RNA of a virus is not defined on the 4th week of treatment. However, at a 16 weeks course of treatment the risk of a recurrence is higher, than at a 24 weeks course of treatment. At patients of the given group at making decision on duration of therapy it is necessary to take shipping of the combined treatment and degree of fibrosis of a liver into consideration. Shortening of duration of treatment at patients with genotypes 2 or 3 with initial high viral load at which RNA of a virus is not defined on the 4th week, it is necessary to allow with bigger care as negative impact of VVN on the steady virologic answer is not excluded.
For patients with genotypes 5 or 6 the 48 weeks course of treatment пегИФНα-2а or 2b in a combination with Rivirin is recommended to a dose of 1000-1200 mg.
Table 1.
NVN, BVO
does not have the recommendation about dosing at the combined treatment of patients with a viral hepatitis S. Genotip of a virus pegifn pegifn Rivirin's Dose in day of Prodolzhitelnost of treatment α-2a α-2b Genotip 1, * 180 mkg 1.5 mkg/kg> 75 kg = 1200 mg of 24 or 48 weeks Genotip 1, VVN, BVO * 180 mkg 1.5 mkg/kg> 75 kg = 1200 mg of 48 weeks Genotip 4, BVI * 180 mkg 1.5 mkg/kg> 75 kg = 1200 mg of 24 or 48 weeks Genotip 1 or 4, BVI *
180
mkg 1.5 mkg/kg
> 75 kg = 1200 mg
of 48 weeks
Genotip 2 or 3, NVN, BVO *
180
mkg 1.5 mkg/kg 800 mg
16 or 24 weeks
Genotip 2 or 3, VVN, BVO *
180
mkg 1.5 mkg/kg 800 mg
24 weeks
Genotip 2 or 3, without BVI *
180
mkg 1.5 mkg/kg 800 mg
24 weeks
of BVI * - prompt virologic reply (RNA of a virus is not defined on the 4th week).
NVN – low viral load (no more than 800000ME/ml),
VVN – high viral load (more than 800000 ME/ml).
Table 2. Calculation of a daily dose of Rivirin.
The body weight (kg)
Rivirin
the Daily dose
(mg)
Quantity
of tablets 40 - 65 kg
800 2 tablets in the morning +2 tablets in the evening
of 66-80 kg
1000 2 tablets in the morning +3 tablets in the evening
81-105kg 1200 3 tablets in the morning +3 tablets in the evening
>
105 1400 3 tablets in the morning +4 tablets in the evening
Table 3. Recommendations about change of a dosage of Rivirin for elimination of the anemia which arose as a result of treatment.
The laboratory
Reduce a Dose by 200 Mg/days values * and/or it is necessary to appoint erythropoietin only if:
Administration of drug should be stopped if **:
Hemoglobin
of 85 g/l
Hemoglobin at patients with heart diseases
decrease more, than on 2 g/dl within any 4 weeks during therapy (continuous reduction of a dose)
* patients to whom Rivirin's dose decreases to 600 mg a day accept on one 200-mg tablet in the morning and two tablets in the evening
** at normalization of indicators it is possible to start over again treatment by Rivirin in a dose of 600 mg a day, further increase in a dose up to 800 mg a day – to the discretion of the doctor. Return to higher doses is not recommended.
Chronic hepatitis C at inefficiency of the previous treatment
the Recommended Rivirin's dosage in a combination with пегИФНα-2а makes a day 1000 mg for patients with body weight less than 75 kg and 1200 mg for patients with the body weight of 75 kg and above, irrespective of a genotype, in a combination with пегИФНα-2b the daily dose makes 15мг/кг/сут. The recommended treatment duration for a genotype 1 or 4 is 72 weeks, and 48 weeks – for genotypes 2 or 3.
Use in a combination with standard interferon alfa-2
the Recommended Rivirin's doses at use in a combination with interferon alfa-2 depend on the body weight of the patient.
The body weight of the patient
the Daily dose
of Riverin Prodolzhitelnost of treatment
Quantity of tablets on 200 mg
<>
1000 mg
of 24 or 48 weeks
5 (in the 2nd morning and in the 3rd evening)
> 75
1200 mg
of 24 or 48 weeks
6 (in the 3rd morning and in the 3rd evening)
Prodolzhitelnost of treatment makes not less than 6 months. Patients with a genotype of virus 1 have to receive combination therapy within 48 weeks. For patients with other genotypes of a virus the decision on extension of therapy to 48 weeks has to be based on other predictive factors (such as initially high viral load, a male, age is more senior than 40 years and presence of bridge-like fibrosis of a liver at a histologic research of the bioptat).
Use at the compensated cirrhosis
to Patients with quantity of neutrophils more than 1.5·109/l and thrombocytes > 70·109/l peginterferon and Rivirin are appointed in a standard dose with weekly control of the general blood test.
At emergence of side effects or changes of laboratory indicators during treatment by Rivirin in a combination with пегИФНα-2 or interferon alfa-2 it is necessary to change doses of each of drugs before stopping of side reactions. At preservation of these phenomena after change of a dose of Rivirin, it is necessary to cancel treatment.
At lack of decrease in the HCV RNA level from a reference value on 2 log10 or more (≥100 times) in 12 weeks of treatment PVT should be stopped.
Side effects
Type and frequency of side effects as a result of combination therapy are caused by the known profile of safety of interferon alpha 2а or 2b or пегИФНα-2а or 2b and a ribavirina.
Often
- insomnia, irritability, a depression, loss of concentration of attention, a headache, dizziness
- decrease in body weight
- short wind, cough
- anorexia, nausea, diarrhea, an abdominal pain
- an alopecia, an itching, dermatitis, xeroderma
- myalgia, an arthralgia
- weakness, temperature increase, a fever, an asthenia
Infrequently
- herpes, infections of an urinary path, bronchitis, candidiasis of an oral cavity
- a lymphadenopathy
- anemia, thrombocytopenia
- a hypothyroidism, a hyperthyroidism
- disturbance of memory, paresthesia, a giposteziya, a tremor, weakness, dizziness, emotional lability, nervousness, aggression, decrease a libido, migraine, a hyperesthesia, nightmares, uneasiness, a syncope
- a zatumanennost in eyes, a xerophthalmia, conjunctivitis, eye pain
- ear pain
- heartbeat, tachycardia, inflows, an asthma at physical activity
- rhinitis, nasopharyngites, congestion of a nose, nasal bleeding
- dryness in a mouth, ulcerations of a mucous oral cavity, an odontorrhagia, stomatitis, a dysphagy, a glossitis, disturbance of taste, thirst, vomiting, dyspepsia, a meteorism
- rash, eczema, psoriasis, reactions of photosensitivity
- the increased perspiration, night sweats, peripheral hypostases
- an ostealgia, a stethalgia, in a back and a neck, muscle weakness, spasms of muscles, muscle pain, arthritis
- erectile dysfunction
- a grippopodobny symptom, fatigue
Seldom
- milk acidosis (hyper lactacidemia), the acquired lipodystrophy and a chromaturia
- flu, pneumonia, throat and throat pain, a cheilitis
- affective lability, apathy
- a ring in ears
Very seldom (isolated cases)
- lower respiratory tract infections, skin infections, external otitis
- suicide mood, psychotic disorders, hallucinations, peripheral neuropathy
- an abnormal liver function, fat dystrophy of a liver, a cholangitis, a malignant tumor of a liver
- a peptic ulcer of a stomach, gastrointestinal bleedings, pancreatitis
- arrhythmia, atrial fibrillation, a lowering of arterial pressure, a pericarditis, an endocarditis
- a coma and hemorrhage in a brain
- an embolism of a pulmonary artery
- autoimmune diseases (for example, a thyroiditis, myocarditis, a pseudorheumatism), a miositis, a sarcoidosis, an interstitial pneumonitis with a lethal outcome, a keratohelcosis
- a pancytopenia or aplastic anemia
the Side reactions connected with reception of a ribavirin
- decrease in level of hemoglobin, quantity of erythrocytes
-
Contraindication insomnia
- hypersensitivity to a ribavirin or any component of drug
- a serious illness of heart, including unstable and uncontrollable forms which are observed, at least, for 6 months prior to treatment
- pregnancy and the period of a lactation (therapy should not be begun data on negative take of the test for pregnancy)
- children's and teenage age up to 18 years
- hemoglobinopathies (for example, a thalassemia, a sickemia will not be obtained yet)
- a serious, wearisome illness, including at patients with chronic kidney disease or with clearance of creatinine is lower than 50 ml/min.
- a heavy abnormal liver function or dekompensirovanny cirrhosis
- autoimmune hepatitis or other autoimmune diseases in the anamnesis
- the profound depression and suicide moods
Medicinal interactions
Enzymes of P450 cytochrome do not participate in metabolic transformations of a ribavirin. Therefore there is the minimum probability for interaction with P450 cytochrome.
Antacids. The bioavailability of a ribavirin in a dose of 600 mg decreases at joint intake of the antiacid drug containing compounds of magnesium and aluminum or simetikon.
Analogs of nucleosides. Ribavirin oppresses phosphorylation of a zidovudine and stavudin. Rivirin's use with a zidovudine or stavudiny can lead to increase in concentration of HIV in blood plasma. Therefore careful monitoring of levels of concentration of RNK-VICh in blood plasma at patients who receive treatment by Rivirin in a combination with one of these two means is recommended. At increase in level of concentration of RNK-VICh in plasma, Rivirin's use with inhibitors of reverse transcriptase should be reconsidered.
Use of nukleozidny analogs separately or in a combination with other nucleosides, can lead to development of a lactacidemia. Rivirin increases the maintenance of fosforilirovanny metabolites of purine nucleosides. This effect can exponentiate risk of developing of the lactacidemia caused by purine analogs of nucleosides (for example didanozin or abakavir).
At the HIV-positive patients receiving VAART (highly active antiretroviral therapy) the risk of developing of lactoacidosis increases. Therefore it is necessary to perform carefully combination therapy against the background of VAART.
The possibility of interaction with Rivirin remains for two months (5 elimination half-life of a ribavirin) after phase-out, thanks to long elimination half-life.
The special
instructions Use of a Ribavirin as monotherapy inefficiently therefore Rivirin should not be applied independently at treatment of hepatitis C. Evening administration of drug no later than 19:00 hours as one of side effects is insomnia is recommended.
Hemolysis/anemia
Though drug has no direct impact on a cardiovascular system, the anemia connected with Rivirin's reception can strengthen heart failure and/or provoke aggravation of symptoms of a coronary disease. Therefore therapy by Rivirin in a combination with peginterferon alpha 2а or 2b or interferon alpha 2а or 2b, has to be appointed with care to patients with heart diseases. It is necessary to estimate a condition of a cardiovascular system before an initiation of treatment and during therapy to carry out clinical monitoring, in case of identification of any signs of deterioration from a cardiovascular system the therapy has to be stopped.
Owing to hemolysis the level of uric acid in blood serum can increase. Therefore, considering, risk of developing gout needs to be watched the patients predisposed to this disease carefully.
A cardiovascular system
Adult patients who have signs (or in the anamnesis) stagnant heart failure, a myocardial infarction and/or arrhythmia, have to be under constant observation of the doctor. At patients with heart diseases before the beginning and during treatment it is recommended to carry out electrocardiography. Arrhythmias (generally supraventricular), as a rule, give in to traditional therapy, but can demand the therapy termination.
Immediate hypersensitivity
At development of acute reaction of hypersensitivity (for example, small tortoiseshells, a Quincke's disease, a bronchospasm, an anaphylaxis) Rivirin's use it is necessary to stop and appoint the corresponding treatment immediately. Tranzitorny rashes are not the basis for the treatment termination.
Use in an abnormal liver function
of Pharmacokinetic interaction between ribaviriny and function of a liver is not revealed. Therefore for patients with a liver failure the modification of a dose of Rivirin in a combination with peginterferon alpha 2а or 2b or interferon alpha 2а or 2b is not required. Therapy should be stopped in case of progressing of signs and symptoms of an abnormal liver function. Rivirin in a combination with peginterferon alpha 2а or 2b or interferon alpha 2а or 2b is contraindicated in heavy dysfunction of a liver or in a dekompensirovanny disease of a liver.
Hepatotoxicity, including fatal, was observed seldom at use of interferon alpha 2а or 2b. At use of a ribavirin in a combination with interferon alpha 2а or 2b for the patient it is necessary to establish careful observation and control of laboratory indicators. If ALT (alaninaminotranspherase) is doubled up to the size or more exceeding a reference value, treatment can be continued in the absence of symptoms of a liver failure. At the same time, definition of ALT, nuclear heating plant (aspartate aminotransferase), a prothrombin time, alkaline phosphatase, albumine and bilirubin should be carried out every 2 week.
Use in a renal failure
Due to the considerable decrease in clearance of creatinine at patients with renal dysfunction, Rivirin's pharmacokinetics at such patients is broken. Therefore it is recommended to estimate function of kidneys at all patients prior to therapy by Rivirin. Patients with clearance of creatinine should not apply lower than 50 ml/min. Rivirin.
Use for elderly people (³ 65 years)
Is recommended to estimate function of kidneys prior to therapy.
Mental disturbances and the central nervous system
If it is decided that combination therapy with Rivirin is necessary for patients with the clinical or anamnestic data confirming heavy mental disturbance it should be begun only after performing the corresponding individual diagnostics and against the background of therapeutic maintaining a mental state. At development of heavy neuropsychiatric effects, especially depressions, Rivirin's use in a combination with peginterferon alpha 2а or 2b or interferon alpha 2а or 2b it is necessary to interrupt.
At emergence of mental disturbances or changes from central nervous system including clinic of a depression it is recommended to watch patients constantly during treatment and during further observation considering potential gravity of the similar undesirable phenomena. At preservation or increase of symptoms, or at identification of thoughts of a suicide or the agressive behavior directed on people around it is recommended to stop treatment and to render to the patient the appropriate mental health services.
Use at to - infection of HIV and hepatitis C virus
At the patients receiving therapy by nenukleozidny inhibitors of reverse transcriptase in a complex with a combination of a ribavirin and peginterferon alpha 2а or 2b/interferon alpha 2а or 2b can increase risk of development of mitochondrial toxicity, lactoacidosis and liver failure.
At to - the infected patients with cirrhosis receiving highly active antiretroviral therapy (VAART) risk of development of a hepatic decompensation and death increases. Additional appointment the alpha of interferon separately or in a combination with ribaviriny increases the above-stated risks at this category of patients.
Treatment of patients with the low level of CD of 4+ cells needs to be carried out carefully.
Dental and periodontal disturbances
the Dryness in a mouth can render the damaging effect on teeth and a mucous membrane of a mouth during long combination therapy by Rivirin, interferon alpha 2а or 2b or peginterferon alpha 2а or 2b. Patients should recommend to brush carefully teeth 2 times a day and to regularly undergo dental inspection. Besides, some patients can have a vomiting then they have to rinse an oral cavity carefully.
Laboratory researches.
All patients prior to therapy and during treatment (on the 2nd and 4th week and then as necessary) are recommended to carry out the general blood test (the developed analysis with definition of a leukocytic formula) and biochemical analysis of blood (electrolytes, serumal creatinine, hepatic tests, uric acid), a research of function of a thyroid gland.
The women of childbearing age
of the Woman receiving treatment, and women – sexual partners receiving treatment, have to monthly throughout the entire period of treatment and 6 months after completion of treatment to carry out tests for pregnancy.
Control of function of a thyroid gland
before PVT needs to be defined the level of thyroid stimulating hormone (TSH) and existence of antibodies to thyreoglobulin (TG) and thyroid peroxidase (TPO). Any deviations of thyroid function found at this time have to be modified by traditional therapy. If the content of TSH manages to be supported by medicamentous therapy at the normal level, antiviral treatment can be begun. At emergence of symptoms of dysfunction of a thyroid gland against the background of treatment it is necessary to determine by alpha interferon the thyroid status and to carry out the corresponding treatment.
Embriotoksichesky and teratogenic action
Ribavirin collects intracellularly and is brought out of an organism very slowly, therefore, can collect in sperm and show inherent in it teratogenic or genotoksichesky actions on a human embryo / fruit.
The feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
during treatment to the persons feeling fatigue, drowsiness or a disorientation needs to abstain from driving of motor transport and occupations potentially dangerous types of activity demanding the increased concentration of attention and speed of psychomotor reactions.
Overdose
Symptoms: strengthening of expressiveness of side effect is possible.
Treatment: drug withdrawal, symptomatic therapy.
A form of release and packing
On 10 tablets in blister strip packaging from a film of polyvinylchloride and aluminum foil.
On the 3rd blister strip packagings together with the instruction for medical use in the state and Russian languages place in a pack from cardboard.
To Store storage conditions in the dry, protected from light place at a temperature not over 250C.
To store out of children's reach!
A period of storage
2 years
not to use drug after the expiry date specified on packing.
Prescription status
According to the prescription
JV Global Pharm LLP Producer,
Republic of Kazakhstan. Almaty, Dzhandosov St. 184 of , ph. 309-74-07, 309-74-14.
The address of the organization of the claim accepting in the territory of the Republic of Kazakhstan from the consumer on quality of products:
JV Global Pharm LLP
050042, Republic of Kazakhstan, Almaty, Dzhandosov St. 184 of
ph. 309-74 - 07, fax 309-74-14. e-mail: globalzavod@mail.ru.
To develop