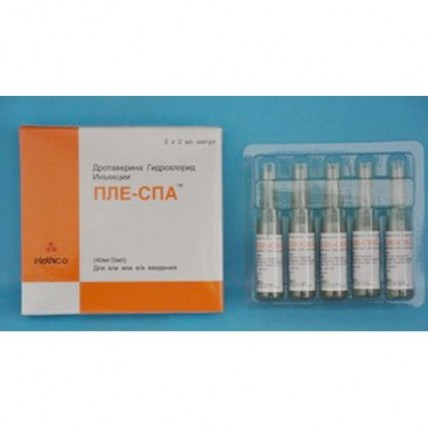
Ple-spa 40 mg / 2 ml 5's solution for injection in ampoules
- $6.00
Out Of Stock
The instruction for medical use
of PLE-SPA medicine
the Trade name
of Ple-Spa
the International unlicensed
name Drotaverinum Dosage Form Solution for injections for intramuscular and intravenous administration, 40 mg / 2 ml.
Ingredients:
1 ml of solution contains:
Active agent: Drotaverinum hydrochloride of 20 mg,
Excipients: water for injections, marked paraben, spent on drink paraben, dinatrium editat, propylene glycol, dinatrium metabisulphite, ethanol.
The description
Transparent solution of yellow color
the Pharmacotherapeutic
Antispasmodic Code of Automatic Telephone Exchange A03AD02 Pharmacological Pharmacokinetics Way Properties group of introduction does not influence hydrochloride Drotaverinum pharmacokinetics.
After introduction of a single intravenous dose of 80 mg average area under a curve the plasma concentration time (AUC) makes 3251 ng/ml an hour. Elimination half-life of Drotaverinum fluctuates from 7 to 12 hours. About 90% of the entered dose are soaked up from a small intestine within 60 minutes. Drotaverinum and its metabolites for 80-95% contact blood proteins. The spasmolytic effect which had by not changed substance comes in 30 minutes in most cases, and lasts for about 4 hours.
Drotaverinum is exposed to extensive metabolism in a liver, drug is removed generally with urine and excrements. The renal clearance is 0.21-1.28 ml a minute.
The pharmacodynamics
of Ple-Spa is the anticholinergic eliminating spasms of smooth muscles of internals.
Spasmolytic effect of drug is mediated by suppression of effect of the enzyme of phosphodiesterase-IV specific to smooth muscles. Drug has fast direct effect on unstriated muscles that is connected with influence on ts-AMF and elimination of a calcic imbalance on the spastic site, and, thus, stops spasms of smooth muscles and pain.
Indications:
- Digestive tract spasms: spasms in a peptic ulcer of a stomach and duodenum, spasms of peloric and cardial department of a stomach, a spastic constipation, spasms in colitis and a meteorism, tenesmus
- Bilious colic and spasms in diseases of a gall bladder, cholelithiasis, cholecystitis
- Renal colic in a nephrolithiasis, pyelonephritis, spastic pains in cystitis and prostatitis
- Spasms of smooth muscles when holding diagnostic procedures, medical termination of pregnancy, dilatation and scraping, a postoperative meteorism of spastic origin
- Algodismenorey and a spasm of a neck of the uterus
- For simplification of patrimonial activity and prevention of ruptures of a neck of the uterus
the Route of administration and doses:
Adult: 40-80 mg in oil 1-3 times a day.
For stopping of acute calculous gripes appoint 40-80 mg in the form of a slow intravenous injection. In diseases of peripheral arteries drug can be appointed vnutriarterialno.
To children from 1 year to 6 years: 20 mg in oil 1-3 times a day.
Children are from 6 to 12 years old: 40 mg in oil 1-3 times a day.
Maximum single dose: 80 mg.
Maximum daily dose: 240 mg.
Side effects
- from a cardiovascular system: hypotension, tachycardia.
- from the central nervous system: headache, dizziness.
- seldom: ruptures of a neck of the uterus
- it is rare:
Contraindication nausea
- hypersensitivity
- glaucoma
- the profound atherosclerosis of coronary arteries
- prostate adenoma
- heavy renal, liver and heart failure
Medicinal interactions
Drotaverinum can safely be applied to Drotaverinum with other drugs. It is necessary to show care at simultaneous use with drug a levodopa in parkinsonism as the antiparkinsonichesky effect decreases.
Simultaneous use with Drotaverinum of anesthetics, antimuskarinovy means or benzodiazepines leads to strengthening of their action.
Special
instructions Pregnancy and period of a lactation:
It is unknown of effect of drug during pregnancy at the person. Data on use of drug for pregnant women are not enough therefore pregnant women should be careful when prescribing Drotaverinum.
There are no messages about use of Drotaverinum in the period of a lactation. If necessary treatment has to be carried out only under medical observation.
Patients with a renal failure or a liver:
Dose adjustment at patients with a renal failure or a liver as Drotaverinum and its metabolites are exposed to extensive metabolism in a liver is necessary and are removed through kidneys.
Influence of drug on ability of driving of transport or work with dangerous mechanisms
About influence it was not reported.
Overdose
of Data on overdose there is no drug.
At accidental overdose appoint gastric lavage and activated carbon inside. At emergence of any symptoms the symptomatic treatment is shown.
Form of release and packing:
Primary packing: ampoule of 2 ml.
Secondary packing: in cardboard packing of 5 ampoules with the instruction for use.
Storage conditions:
To store in the place protected from light at a temperature not over 25C.
To store out of children's reach!
3 years
not to apply a period of storage after an expiration date.
Prescription status
According to the prescription
the Producer:
Plethico Pharmaceuticals Ltd/Pletkhiko Pharmasyyutikalz Ltd
location Address:
Dharavara, Kalariya - 453001, Indore (L. S.), India
the Legal address:
37/37A, Indastrial Isteyt, Polograund, Indore (L. S.), 452,015, India
of PLE-SPA medicine
the Trade name
of Ple-Spa
the International unlicensed
name Drotaverinum Dosage Form Solution for injections for intramuscular and intravenous administration, 40 mg / 2 ml.
Ingredients:
1 ml of solution contains:
Active agent: Drotaverinum hydrochloride of 20 mg,
Excipients: water for injections, marked paraben, spent on drink paraben, dinatrium editat, propylene glycol, dinatrium metabisulphite, ethanol.
The description
Transparent solution of yellow color
the Pharmacotherapeutic
Antispasmodic Code of Automatic Telephone Exchange A03AD02 Pharmacological Pharmacokinetics Way Properties group of introduction does not influence hydrochloride Drotaverinum pharmacokinetics.
After introduction of a single intravenous dose of 80 mg average area under a curve the plasma concentration time (AUC) makes 3251 ng/ml an hour. Elimination half-life of Drotaverinum fluctuates from 7 to 12 hours. About 90% of the entered dose are soaked up from a small intestine within 60 minutes. Drotaverinum and its metabolites for 80-95% contact blood proteins. The spasmolytic effect which had by not changed substance comes in 30 minutes in most cases, and lasts for about 4 hours.
Drotaverinum is exposed to extensive metabolism in a liver, drug is removed generally with urine and excrements. The renal clearance is 0.21-1.28 ml a minute.
The pharmacodynamics
of Ple-Spa is the anticholinergic eliminating spasms of smooth muscles of internals.
Spasmolytic effect of drug is mediated by suppression of effect of the enzyme of phosphodiesterase-IV specific to smooth muscles. Drug has fast direct effect on unstriated muscles that is connected with influence on ts-AMF and elimination of a calcic imbalance on the spastic site, and, thus, stops spasms of smooth muscles and pain.
Indications:
- Digestive tract spasms: spasms in a peptic ulcer of a stomach and duodenum, spasms of peloric and cardial department of a stomach, a spastic constipation, spasms in colitis and a meteorism, tenesmus
- Bilious colic and spasms in diseases of a gall bladder, cholelithiasis, cholecystitis
- Renal colic in a nephrolithiasis, pyelonephritis, spastic pains in cystitis and prostatitis
- Spasms of smooth muscles when holding diagnostic procedures, medical termination of pregnancy, dilatation and scraping, a postoperative meteorism of spastic origin
- Algodismenorey and a spasm of a neck of the uterus
- For simplification of patrimonial activity and prevention of ruptures of a neck of the uterus
the Route of administration and doses:
Adult: 40-80 mg in oil 1-3 times a day.
For stopping of acute calculous gripes appoint 40-80 mg in the form of a slow intravenous injection. In diseases of peripheral arteries drug can be appointed vnutriarterialno.
To children from 1 year to 6 years: 20 mg in oil 1-3 times a day.
Children are from 6 to 12 years old: 40 mg in oil 1-3 times a day.
Maximum single dose: 80 mg.
Maximum daily dose: 240 mg.
Side effects
- from a cardiovascular system: hypotension, tachycardia.
- from the central nervous system: headache, dizziness.
- seldom: ruptures of a neck of the uterus
- it is rare:
Contraindication nausea
- hypersensitivity
- glaucoma
- the profound atherosclerosis of coronary arteries
- prostate adenoma
- heavy renal, liver and heart failure
Medicinal interactions
Drotaverinum can safely be applied to Drotaverinum with other drugs. It is necessary to show care at simultaneous use with drug a levodopa in parkinsonism as the antiparkinsonichesky effect decreases.
Simultaneous use with Drotaverinum of anesthetics, antimuskarinovy means or benzodiazepines leads to strengthening of their action.
Special
instructions Pregnancy and period of a lactation:
It is unknown of effect of drug during pregnancy at the person. Data on use of drug for pregnant women are not enough therefore pregnant women should be careful when prescribing Drotaverinum.
There are no messages about use of Drotaverinum in the period of a lactation. If necessary treatment has to be carried out only under medical observation.
Patients with a renal failure or a liver:
Dose adjustment at patients with a renal failure or a liver as Drotaverinum and its metabolites are exposed to extensive metabolism in a liver is necessary and are removed through kidneys.
Influence of drug on ability of driving of transport or work with dangerous mechanisms
About influence it was not reported.
Overdose
of Data on overdose there is no drug.
At accidental overdose appoint gastric lavage and activated carbon inside. At emergence of any symptoms the symptomatic treatment is shown.
Form of release and packing:
Primary packing: ampoule of 2 ml.
Secondary packing: in cardboard packing of 5 ampoules with the instruction for use.
Storage conditions:
To store in the place protected from light at a temperature not over 25C.
To store out of children's reach!
3 years
not to apply a period of storage after an expiration date.
Prescription status
According to the prescription
the Producer:
Plethico Pharmaceuticals Ltd/Pletkhiko Pharmasyyutikalz Ltd
location Address:
Dharavara, Kalariya - 453001, Indore (L. S.), India
the Legal address:
37/37A, Indastrial Isteyt, Polograund, Indore (L. S.), 452,015, India