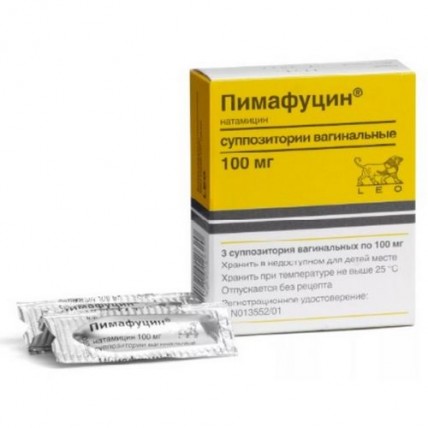
Pimafutsin 3's 100mg Vaginal suppository
- $20.60
The instruction for medical use
of Pimafutsin® medicine
the Trade name
of Pimafutsin®
the International not patent
name Natamitsin Lekarstvennaya a form
Suppositories vaginal, 100 mg
Structure
1 suppository contains
active agent - natamitsin 100 mg,
excipients: cetyl alcohol, fat firm, sorbitan trioleate, polysorbate 80, Natrii hydrocarbonas, adipic acid
Description
Suppositories of a torpedo-shaped form, pale yellow or slightly yellow color.
Pharmacotherapeutic group
of Antiseptics and antimicrobial drugs for treatment of gynecologic diseases (excepting combinations with corticosteroids). Natamitsin.
The ATX G01AA02 code
the Pharmacological
Pharmacokinetics Natamitsin properties – drug of local action, is practically not soaked up through mucous membranes.
Pharmacodynamics
Antifungal half-yen antibiotic of fungicide action. Natamitsin connects sterols of cellular membranes, breaking their integrity and functions that leads to death of microorganisms. The majority of a pathogenic barmy fungi, especially Candida spp are sensitive to a natamitsin. Dermatophytes are less sensitive. The resistance to a natamitsin in clinical practice does not occur among barmy mushrooms.
Indications
- a vaginitis, a vulvitis, a vulvovaginitis
Appoint the Route of administration and doses on 1 suppository within 3-6 days.
Suppositories enter into a vagina in a prone position as it is possible more deeply,
1 time/days for the night.
At a persistent course of the vulvovaginitis caused by Candida albicans in addition appoint tablets inside (on 1 tab. 4 times/days within 7-10 days).
For treatment of candidosis damage of genitals of the partner use Pimafutsin in the form of cream.
Side effects
- the slight irritation, burning sensation
of the Contraindication
- hypersensitivity to drug components
- a porphyria
Medicinal interactions
of Medicinal interactions is not described
Special instructions
Suppositories vaginal under the influence of body temperature quickly are dissolved, forming foamy weight that promotes hypodispersion of active substance. In case of persistent or recurrent infection the topical treatment can be complemented with purpose of tablets and cream.
Ethanol which is a part of suppositories vaginal can cause reactions of hypersensitivity. In the period of periods the therapy with suppositories is interrupted. During treatment by suppositories there is no need for an exception of sexual contacts. However it is recommended to perform examination of sexual partners and, in case of identification of candidosis defeat, to treat with Pimafutsin. Also it is necessary to provide use during treatment of barrier methods of contraception.
Pregnancy and the period of a lactation
Pimafutsin's use during pregnancy and a lactation is possible.
Features of influence of medicine on ability to drive the car or potentially dangerous mechanisms
the Overdose does not influence
Now about cases of overdose of the drug Pimafutsin it was not reported.
The form of release and packing
On 3 suppositories place in blister strip packaging from a film polyvinylchloride.
On 1 blister strip packaging together with the instruction for medical use in the state and Russian languages place in a cardboard pack.
To Store storage conditions at a temperature of 15 - 25 wasps.
To store out of children's reach!
2 years
not to apply a period of storage after the expiration date specified on packing.
Prescription status
According to the prescription
the Producer Temmler Italy Neuter. L.,
Via Industri's 2 business, 20061 Karugat (MI), Italy
Owner of the registration certificate
Astellas of Pharm B.V. Europe, Netherlands.
The address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products
Representation Astellas of Pharm B.V. Europe in PK
PK, Almaty, 050559, Al-Farabi Ave. 15, the Centre Party of Finland of Nurla Tau,
zd 4B, office No. 20
Phone number 8 (727) 311-13-89
Fax 8 (727) 311-13-90
e-mail of astellas@com.kz
of Pimafutsin® medicine
the Trade name
of Pimafutsin®
the International not patent
name Natamitsin Lekarstvennaya a form
Suppositories vaginal, 100 mg
Structure
1 suppository contains
active agent - natamitsin 100 mg,
excipients: cetyl alcohol, fat firm, sorbitan trioleate, polysorbate 80, Natrii hydrocarbonas, adipic acid
Description
Suppositories of a torpedo-shaped form, pale yellow or slightly yellow color.
Pharmacotherapeutic group
of Antiseptics and antimicrobial drugs for treatment of gynecologic diseases (excepting combinations with corticosteroids). Natamitsin.
The ATX G01AA02 code
the Pharmacological
Pharmacokinetics Natamitsin properties – drug of local action, is practically not soaked up through mucous membranes.
Pharmacodynamics
Antifungal half-yen antibiotic of fungicide action. Natamitsin connects sterols of cellular membranes, breaking their integrity and functions that leads to death of microorganisms. The majority of a pathogenic barmy fungi, especially Candida spp are sensitive to a natamitsin. Dermatophytes are less sensitive. The resistance to a natamitsin in clinical practice does not occur among barmy mushrooms.
Indications
- a vaginitis, a vulvitis, a vulvovaginitis
Appoint the Route of administration and doses on 1 suppository within 3-6 days.
Suppositories enter into a vagina in a prone position as it is possible more deeply,
1 time/days for the night.
At a persistent course of the vulvovaginitis caused by Candida albicans in addition appoint tablets inside (on 1 tab. 4 times/days within 7-10 days).
For treatment of candidosis damage of genitals of the partner use Pimafutsin in the form of cream.
Side effects
- the slight irritation, burning sensation
of the Contraindication
- hypersensitivity to drug components
- a porphyria
Medicinal interactions
of Medicinal interactions is not described
Special instructions
Suppositories vaginal under the influence of body temperature quickly are dissolved, forming foamy weight that promotes hypodispersion of active substance. In case of persistent or recurrent infection the topical treatment can be complemented with purpose of tablets and cream.
Ethanol which is a part of suppositories vaginal can cause reactions of hypersensitivity. In the period of periods the therapy with suppositories is interrupted. During treatment by suppositories there is no need for an exception of sexual contacts. However it is recommended to perform examination of sexual partners and, in case of identification of candidosis defeat, to treat with Pimafutsin. Also it is necessary to provide use during treatment of barrier methods of contraception.
Pregnancy and the period of a lactation
Pimafutsin's use during pregnancy and a lactation is possible.
Features of influence of medicine on ability to drive the car or potentially dangerous mechanisms
the Overdose does not influence
Now about cases of overdose of the drug Pimafutsin it was not reported.
The form of release and packing
On 3 suppositories place in blister strip packaging from a film polyvinylchloride.
On 1 blister strip packaging together with the instruction for medical use in the state and Russian languages place in a cardboard pack.
To Store storage conditions at a temperature of 15 - 25 wasps.
To store out of children's reach!
2 years
not to apply a period of storage after the expiration date specified on packing.
Prescription status
According to the prescription
the Producer Temmler Italy Neuter. L.,
Via Industri's 2 business, 20061 Karugat (MI), Italy
Owner of the registration certificate
Astellas of Pharm B.V. Europe, Netherlands.
The address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products
Representation Astellas of Pharm B.V. Europe in PK
PK, Almaty, 050559, Al-Farabi Ave. 15, the Centre Party of Finland of Nurla Tau,
zd 4B, office No. 20
Phone number 8 (727) 311-13-89
Fax 8 (727) 311-13-90
e-mail of astellas@com.kz