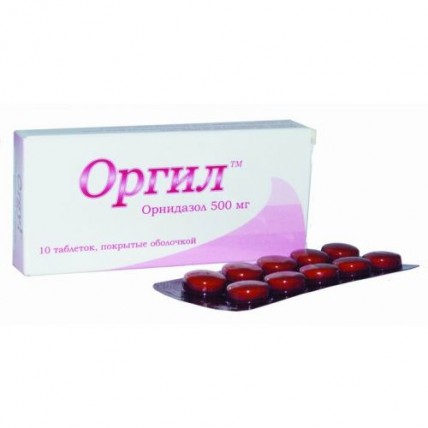
Orgil 10s ™ 500 mg coated tablets
- $14.80
The instruction for medical use
of ORGIL™ medicine
the Trade name
of Orgil™
the International unlicensed
name Ornidazol Lekarstvennaya
the Tablet form, coated 500 mg
Structure
One tablet contains
active agent - ornidazol 500 mg,
excipients: microcrystalline cellulose, starch, sodium of a kroskarmelloz, magnesium stearate
structure of a cover: Opadray 03B53217 orange *
*-structure of a cover: FD&C yellow No. 6 aluminum varnish (AP0072), polyethyleneglycol, FD&C yellow No. 6 aluminum varnish (AP0024), gipromelloz, titan dioxide (E 171).
Description
of the Tablet, coated orange color, round shape.
Pharmacotherapeutic group
Drugs for treatment of an amebiasis and other protozoan infections. Nitroimidazole derivatives. Ornidazol
the ATX P01AB03 Code
the Pharmacological
Ornidazol Pharmacokinetics properties is easily and quickly soaked up at intake. The bioavailability is 90%. The maximum concentration in plasma is reached in 3 hours after reception. Linking with proteins makes about 13%. Gets into cerebrospinal fluid, other liquids and into body tissues. Ornidazol is metabolized in a liver. Elimination half-life makes 13 hours. With urine about 63%, through intestines are removed – to 22% of drug.
A pharmacodynamics
of Orgil™ – antiprotozoan means, derivative 5 nitroimidazoles. Has high efficiency at treatment of protozoan and bacterial infections. Entomoeba Histolytica, Giardia lamblia (Giardia intestinalis) and also some anaerobic bacteria, such as Bacteroides spp., and Clostridium spp., Fusobacterium spp., and anaerobic cocci is active concerning Trichomonas vaginalis.
Indications
- trichomoniasis (a mecotic vulvovaginitis, an urethritis, a bartholinitis, a cervicitis, prostatitis, an adnexitis)
- an amebiasis (amoebic dysentery, an abenteric amebiasis, including amoebic abscess of a liver)
- a giardiasis
- prevention of infections after surgical and gynecologic interventions
accept the Route of administration and doses of the Tablet Orgil™ after a meal.
Trichomoniasis: on 500 mg (1 tablet) 2 times a day (in the morning and in the evening) within 5 days or once 1.5 g (3 tablets) in the evening. It is recommended to apply vaginal tablets to achievement of antibacterial effect in addition.
To avoid repeated infection, it is necessary to treat at the sexual partner.
At an intestinal amebiasis: - 1.5 (3 tablets) 1 time a day, at body weight more than 60 kg - 2 g/days (4 tablets: 2 tablets in the morning and 2 tablets in the evening). A course of treatment - 3 days.
At an abenteric amebiasis: - on 0.5 g (1 tablet) inside, in the morning and in the evening within 5-10 days.
In a giardiasis: - inside, on 1.5 g/days (3 tablets). A course of treatment - 1-2 days.
Prevention of the infections caused by anaerobic bacteria: Before operation on 0.5-1g (1-2 tablets), after operation - on 1 tablet 2 times a day within 3-5 days.
Side effects
- dyspepsia, dysfunction of a GIT (including nausea), the perversion of flavoring feelings
- allergic reactions (skin rash, an itching)
is rare:
- the polyuria (as angiotensin can partially block II receptors)
- drowsiness, a headache, dizziness, fatigue, a temporary loss of consciousness, damages of central nervous system (ataxy, a dysarthtia, a tremor, spasms) and peripheral nerves (burning sensation, numbness of extremities, paresthesias)
- oppression of a leukopoiesis
- change of activity of hepatic enzymes, allergic reactions
of the Contraindication
- hypersensitivity to drug
- diseases of the central nervous system (epilepsy,
a brain damage, multiple sclerosis)
- pregnancy and the period of a lactation
- children's age up to 18 years
Medicinal interactions
of Orgil™ enhances effect of anticoagulants of a coumarinic row, prolongs myorelaxation action a bromide vekuroniya
At combined use with other derivatives 5 nitroimidazoles the peripheral neuropathy, a depression epileptoformny spasms can be observed.
Ornidazol does not inhibit an aldegiddegidrogenaza and therefore does not interact with alcohol.
Simultaneous use with inductors of microsomal enzymes (barbiturates, benzodiazepines, rifampitsina, etc.) reduces a half-life period of an ornidazol in serum. Inhibitors of microsomal enzymes (Cimetidinum, macroleads, etc.) increase a half-life period of an ornidazol.
The combination of Orgil™ with neurotoxic and gematoksichny drugs
Special instructions is not recommended
At treatment of trichomoniasis it is necessary to treat both partners.
At heavy insufficiency of function of kidneys and also at patients of advanced age, in connection with age insufficiency of function of a liver it is necessary to provide decrease in dosages.
With caution it is necessary to take at patients with diseases of the central nervous system (in organic diseases and tendency to convulsive reactions), and in diseases of the haematogenic system, a liver, alcoholism.
The feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
Should consider possible reduction in the rate of reaction at control of vehicles, work with the mechanisms demanding accuracy and speed of reaction.
Overdose
Symptoms: spasms, depressive reactions, peripheral neuritis
symptomatic treatment. Antidote is unknown. In spasms – diazepam.
A form of release and packing
On 10 tablets in blister strip packaging from a film of polyvinylchloride and aluminum foil.
On 1 or 10 blister strip packagings together with the instruction for medical use in the state and Russian languages place in a cardboard box.
To Store storage conditions in the dry, protected from light place, at a temperature not above 25 °C.
To store out of children's reach!
3 years
not to use a period of storage after the termination of the expiration date specified on packing.
Prescription status
According to the prescription
the Producer Kusum Heltker Pvt.
Ltd., JV 289 (A), Riiko Indl. Chopanki's Area, Bkhivadi (Raj.), India
Owner of the registration certificate
Kusum Heltker Pvt. Ltd., India
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products: Representative office of Kusum Heltker Pvt. Ltd. Association, India in the Republic of Kazakhstan, legal address: Almaty, Dostyk Ave., 117/6, BC Khan Tengri the location address: Almaty, Dostyk Ave., 117/6, BC Khan Tengri. Phone number: 295-26-50, 295-26-51 (54), Fax: 295-26-55 E-mail: Office@kusumhealthcare.kz
of ORGIL™ medicine
the Trade name
of Orgil™
the International unlicensed
name Ornidazol Lekarstvennaya
the Tablet form, coated 500 mg
Structure
One tablet contains
active agent - ornidazol 500 mg,
excipients: microcrystalline cellulose, starch, sodium of a kroskarmelloz, magnesium stearate
structure of a cover: Opadray 03B53217 orange *
*-structure of a cover: FD&C yellow No. 6 aluminum varnish (AP0072), polyethyleneglycol, FD&C yellow No. 6 aluminum varnish (AP0024), gipromelloz, titan dioxide (E 171).
Description
of the Tablet, coated orange color, round shape.
Pharmacotherapeutic group
Drugs for treatment of an amebiasis and other protozoan infections. Nitroimidazole derivatives. Ornidazol
the ATX P01AB03 Code
the Pharmacological
Ornidazol Pharmacokinetics properties is easily and quickly soaked up at intake. The bioavailability is 90%. The maximum concentration in plasma is reached in 3 hours after reception. Linking with proteins makes about 13%. Gets into cerebrospinal fluid, other liquids and into body tissues. Ornidazol is metabolized in a liver. Elimination half-life makes 13 hours. With urine about 63%, through intestines are removed – to 22% of drug.
A pharmacodynamics
of Orgil™ – antiprotozoan means, derivative 5 nitroimidazoles. Has high efficiency at treatment of protozoan and bacterial infections. Entomoeba Histolytica, Giardia lamblia (Giardia intestinalis) and also some anaerobic bacteria, such as Bacteroides spp., and Clostridium spp., Fusobacterium spp., and anaerobic cocci is active concerning Trichomonas vaginalis.
Indications
- trichomoniasis (a mecotic vulvovaginitis, an urethritis, a bartholinitis, a cervicitis, prostatitis, an adnexitis)
- an amebiasis (amoebic dysentery, an abenteric amebiasis, including amoebic abscess of a liver)
- a giardiasis
- prevention of infections after surgical and gynecologic interventions
accept the Route of administration and doses of the Tablet Orgil™ after a meal.
Trichomoniasis: on 500 mg (1 tablet) 2 times a day (in the morning and in the evening) within 5 days or once 1.5 g (3 tablets) in the evening. It is recommended to apply vaginal tablets to achievement of antibacterial effect in addition.
To avoid repeated infection, it is necessary to treat at the sexual partner.
At an intestinal amebiasis: - 1.5 (3 tablets) 1 time a day, at body weight more than 60 kg - 2 g/days (4 tablets: 2 tablets in the morning and 2 tablets in the evening). A course of treatment - 3 days.
At an abenteric amebiasis: - on 0.5 g (1 tablet) inside, in the morning and in the evening within 5-10 days.
In a giardiasis: - inside, on 1.5 g/days (3 tablets). A course of treatment - 1-2 days.
Prevention of the infections caused by anaerobic bacteria: Before operation on 0.5-1g (1-2 tablets), after operation - on 1 tablet 2 times a day within 3-5 days.
Side effects
- dyspepsia, dysfunction of a GIT (including nausea), the perversion of flavoring feelings
- allergic reactions (skin rash, an itching)
is rare:
- the polyuria (as angiotensin can partially block II receptors)
- drowsiness, a headache, dizziness, fatigue, a temporary loss of consciousness, damages of central nervous system (ataxy, a dysarthtia, a tremor, spasms) and peripheral nerves (burning sensation, numbness of extremities, paresthesias)
- oppression of a leukopoiesis
- change of activity of hepatic enzymes, allergic reactions
of the Contraindication
- hypersensitivity to drug
- diseases of the central nervous system (epilepsy,
a brain damage, multiple sclerosis)
- pregnancy and the period of a lactation
- children's age up to 18 years
Medicinal interactions
of Orgil™ enhances effect of anticoagulants of a coumarinic row, prolongs myorelaxation action a bromide vekuroniya
At combined use with other derivatives 5 nitroimidazoles the peripheral neuropathy, a depression epileptoformny spasms can be observed.
Ornidazol does not inhibit an aldegiddegidrogenaza and therefore does not interact with alcohol.
Simultaneous use with inductors of microsomal enzymes (barbiturates, benzodiazepines, rifampitsina, etc.) reduces a half-life period of an ornidazol in serum. Inhibitors of microsomal enzymes (Cimetidinum, macroleads, etc.) increase a half-life period of an ornidazol.
The combination of Orgil™ with neurotoxic and gematoksichny drugs
Special instructions is not recommended
At treatment of trichomoniasis it is necessary to treat both partners.
At heavy insufficiency of function of kidneys and also at patients of advanced age, in connection with age insufficiency of function of a liver it is necessary to provide decrease in dosages.
With caution it is necessary to take at patients with diseases of the central nervous system (in organic diseases and tendency to convulsive reactions), and in diseases of the haematogenic system, a liver, alcoholism.
The feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
Should consider possible reduction in the rate of reaction at control of vehicles, work with the mechanisms demanding accuracy and speed of reaction.
Overdose
Symptoms: spasms, depressive reactions, peripheral neuritis
symptomatic treatment. Antidote is unknown. In spasms – diazepam.
A form of release and packing
On 10 tablets in blister strip packaging from a film of polyvinylchloride and aluminum foil.
On 1 or 10 blister strip packagings together with the instruction for medical use in the state and Russian languages place in a cardboard box.
To Store storage conditions in the dry, protected from light place, at a temperature not above 25 °C.
To store out of children's reach!
3 years
not to use a period of storage after the termination of the expiration date specified on packing.
Prescription status
According to the prescription
the Producer Kusum Heltker Pvt.
Ltd., JV 289 (A), Riiko Indl. Chopanki's Area, Bkhivadi (Raj.), India
Owner of the registration certificate
Kusum Heltker Pvt. Ltd., India
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products: Representative office of Kusum Heltker Pvt. Ltd. Association, India in the Republic of Kazakhstan, legal address: Almaty, Dostyk Ave., 117/6, BC Khan Tengri the location address: Almaty, Dostyk Ave., 117/6, BC Khan Tengri. Phone number: 295-26-50, 295-26-51 (54), Fax: 295-26-55 E-mail: Office@kusumhealthcare.kz