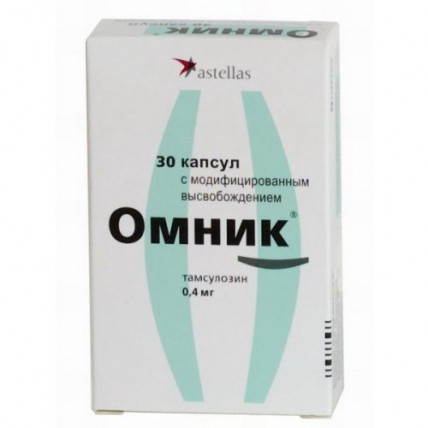
Omnic 0.4 mg 30s modified release capsules
- $38.10
The instruction for medical use
of medicine
Omnik
A trade name
Omnik
Mezhdunarodnoye the unlicensed
name Tamsulozin Lekarstvennaya
the Capsule form with the modified release, 0.4 mg
Structure
One capsule contains
active agent of a tamsulozin a hydrochloride of 0.4 mg,
excipients: microcrystalline cellulose, a kopolimer of methacrylic acid - ethyl acrylate (1:1), polysorbate 80, sodium lauryl sulfate, triacetin, calcium stearate, talc
structure of a cover of gelatin capsules:
structure of a lid indigo carmine (E172), ferrous oxide red (E172), ferrous oxide yellow (E172), the titan dioxide (E171), gelatin
structure of the body - ferrous oxide red (E172), ferrous oxide yellow (E172), the titan dioxide (E171), gelatin
The description
Solid gelatin capsules of size No. 2 with the body of orange color and a lid of olive-green color, with marking 701 and the graphic representation of the trademark on the body and 0.4 on a lid.
Contents of capsules - granules of yellow-white or pale yellow color
Pharmacotherapeutic group
Drugs for treatment of urological diseases. Drugs for treatment of a benign hypertrophy of a prostate. Alpha adrenoblockers. Tamsulozin
ATX G04CA02 Code
Pharmacological
Pharmacokinetics Absorption properties:
Tamsulozin is soaked up in a small intestine, the bioavailability of drug is close to 100%. The concomitant use with food reduces bioavailability of a tamsulozin.
It is possible to provide constancy of conditions of absorption of drug, having recommended to the patient to accept tamsulozin after a breakfast or the first meal.
Pharmacokinetic indicators of a tamsulozin have linear character. After single dose of a tamsulozin on an empty stomach, peak plasma concentration of a tamsulozin is reached approximately in 6 hours, after achievement of an equilibrium state what requires about 5 days of multiple dose of drug, Cmax at patients is approximately two third higher, than is reached at single dose of drug. In spite of the fact that this pattern is noted at elderly people, it is expected that administration of drug by young people, will be followed by similar changes.
The significant individual distinctions are characteristic of plasma concentration of drug after single and multiple dose.
Distribution:
In an organism tamsulozin approximately for 99% contacts proteins of plasma.
Biotransformation:
Tamsulozin has low effect of the first passing. The most part of a tamsulozin is present at plasma in not changed look. Drug is metabolized in a liver.
Any of metabolites has no activity exceeding activity of the most active ingredient of drug. At insignificant and moderate degree of a liver failure the correction of the mode of dosing is not required.
Removal:
Tamsulozin and his metabolites are mainly allocated with urine, at the same time the share of not changed drug makes about 9%. It was established that after single dose of a tamsulozin after meal, and after achievement of an equilibrium state, time of semi-removal of drug is about 10 and 13 hours, respectively.
The volume of distribution of drug is about 0.2 l/kg.
The pharmacodynamics
Tamsulozin active ingredient of the drug Omnik is a selection competitive inhibitor of alfa1-adrenoceptors, drug has affinity to alfa1a and alfa1d to subtypes, linking of a tamsulozin with these receptors relaxes unstriated muscles of a prostate and an urethra.
Omnik increases the maximum flow rate of urine. Reduces obstruction by relaxation of smooth muscles in a prostate and an urethra, thereby facilitating urination disturbance symptoms.
Besides, drug reduces ischuria symptoms in which an important role is played by instability of a bladder.
These effects concerning symptoms of an ischuria and disturbance of urination remain at long therapy. Performing surgical treatment or catheterization is considerably deferred.
Antagonists of 1 adrenoceptors can reduce arterial blood pressure by reduction of peripheric vascular resistance. In the researches Omnika clinically significant lowering of arterial pressure is noted. At a tamsulozin genotoksichesky properties were not revealed.
Indications
- treatment of dysuric disorders in the benign hyperplasia of a prostate (BHP)
the Route of administration and doses
Inside, on 1 capsule after a breakfast. To accept capsules entirely, washing down with water. The capsule is not recommended to be chewed as it can affect drug release speed.
The course of treatment is defined individually by the doctor.
Side effects
Often (& gt, 1/100, & lt, 1/10)
- dizziness
- disturbances of an ejaculation
Infrequently (& gt, 1/1,000, & lt, 1/100)
- a headache
- tachycardia
- orthostatic hypotension
- rhinitis
- nausea, vomiting, a constipation, diarrhea
- rash, a skin itching, urticaria
- an asthenia
Seldom (& gt, 1/10,000, & lt, 1/1,000)
- a syncope
- a Quincke's disease
Very seldom (& lt, 1/10,000) - Stephens-Johnson's Syndrome - a priapism during operation for a cataract was noted development of a syndrome of intraoperative instability of an iris of the eye of an eye (syndrome of a narrow pupil) that was noted in post-marketing observations.
The contraindication
- hypersensitivity to active component of drug or to excipients
- orthostatic hypotension (including in the anamnesis) - the profound liver failure
- with care a heavy renal failure (clearance of creatinine & lt, 10 ml/min.)
- children's and teenage age up to 18 years
Medicinal interactions
was Not noted adverse medicinal interactions when assigning a tamsulozin along with atenolol, enalapril or theophylline.
The concomitant use of drug with Cimetidinum leads to increase in concentration of a tamsulozin in blood plasma, and reception with furosemide - to decrease in plasma concentration of drug, however, concentration remain within acceptable level.
In the researches in vitro with use of microsomes of a liver (reflect the processes happening to participation of isoforms of P450 cytochrome) the interaction at the level of hepatic metabolism with amitriptyline, salbutamol, glibenclamide and finasteridy was not revealed. However, diclofenac and warfarin can increase the clearance rate of a tamsulozin.
At co-administration with the drugs reducing arterial blood pressure, for example, drugs for the general anesthesia or other antagonists of 1 adrenoceptors, increase in their hypotensive action can be noted.
Simultaneous use of a tamsulozin with CYP3A4 inhibitors can lead to strengthening of effect of a tamsulozin. Co-administration of a ketokonazol (known strong CYP3A4 inhibitor) led to increase in AUC and Cmax of a tamsulozin respectively in 2.8 and 2.2 times.
Tamsulozin should not be applied in a combination with the expressed CYP3A4 inhibitors at patients of slow metabolizator with a phenotype of CYP2D6.
Tamsulozin it is necessary to use with care in a combination with the expressed and moderate CYP3A4 inhibitors.
Co-administration of a tamsulozin with paroksetiny, the expressed CYP2D6 inhibitor, was followed increase in Cmax and AUC of a tamsulozin respectively in 1.3 and 1.6 times, however these changes are regarded as clinically not significant.
Special instructions
As well as when using other antagonists of 1 adrenoceptors, in some cases treatment by Omnik can be followed by a lowering of arterial pressure, as a result, in rare instances perhaps development of an unconscious state. At the first symptoms of orthostatic hypotonia (dizziness, weakness) the patient should pass into a sitting position or lying before disappearance of symptoms.
Before an initiation of treatment Omnik it is necessary to perform examination of the patient for an exception of other states, the followed similar symptoms with a benign hyperplasia of a prostate. Before an initiation of treatment and subsequently through regular intervals it is necessary to conduct a manual rectal research and if it is necessary, definition of prostatspetsifichesky antigen (DOG).
Treatment of patients with a heavy renal failure (clearance of creatinine & lt, 10 ml/min.) has to be performed with care as researches at such patients were not conducted.
At the certain patients who were receiving or earlier accepting tamsulozin at operational treatment concerning a cataract or glaucoma the intraoperative syndrome of a flabby iris (Intraoperative Floppy Iris Syndrome, IFIS), option of a syndrome of a narrow pupil was observed. Existence of IFIS can increase risk of complications from eyes in time and after operation.
From experience it is known that there can be useful a cancellation of a tamsulozin of a hydrochloride in 1-2 weeks prior to operation, but effects of such cancellation of therapy are not established yet. Also it was reported about development of IFIS in the patients who stopped reception of a tamsulozin long before operation.
At patients to whom carrying out operational treatment of a cataract or glaucoma is planned, are not recommended to begin therapy of a tamsulozin with a hydrochloride. In process of a preparation for surgery the surgeons and ophthalmologists have to specify, accepts or whether the patient to whom operational treatment of a cataract or glaucoma is planned, tamsulozin accepted earlier to be ready to treatment of IFIS in case of its development during operation.
Pregnancy and the period of a lactation
Drug is intended only for treatment of men. Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms of the Research of influence on ability to driving of the car and to control of mechanisms were not carried out. However, patients have to show care, in connection with a possibility of development of dizziness.
Overdose
Symptoms: heavy arterial hypotension. Treatment: administration of drugs with angiotonic and cardiotonic action is necessary. Transfer of the patient to horizontal position can restore normal arterial blood pressure and heart rate. At inefficiency of these actions carrying out the measures directed to increase in volume of the circulating blood and also introduction of vazopressor is possible. Are necessary assessment of function of kidneys in dynamics and performing maintenance therapy. Because Omnik actively contacts proteins - the hemodialysis is probably inefficient. For treatment of poisonings with high doses of drug performing gastric lavage, intake of activated carbon or osmotic laxatives, such as sodium sulfate is reasonable.
The form of release and packing
On 10 capsules place in blister strip packaging from a film of polyvinylchloride and printing aluminum foil.
On 1 or 3 blister strip packagings together with the instruction for medical use in the state and Russian languages place in a pack from cardboard.
To Store storage conditions at a temperature of 15 - 25 0C.
To store out of children's reach!
4 years
not to apply a period of storage after expiry date.
Prescription status
According to the prescription
the Name and the country
of the Astellas Pharma manufacturing organization Europe B.V., Silviusveg 62, 2333 VE, Leiden, the Netherlands
the Name and the country of the owner of the registration certificate
Astellas of Pharm B.V. Europe, Silviusveg 62, 2333 VE, Leiden, Netherlands
The address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods):
Representation Astellas of Pharm B.V. Europe in RK
Almaty, 050559, Al-Farabi Ave. 15, Nurla Tau's Centre Party of Finland, zd 4B,
office No. 20 Phone number (727) 311-13-89 Fax (727) 311-13-90
www.astellas.ru,
To develop e-mail of astellas@com.kz
of medicine
Omnik
A trade name
Omnik
Mezhdunarodnoye the unlicensed
name Tamsulozin Lekarstvennaya
the Capsule form with the modified release, 0.4 mg
Structure
One capsule contains
active agent of a tamsulozin a hydrochloride of 0.4 mg,
excipients: microcrystalline cellulose, a kopolimer of methacrylic acid - ethyl acrylate (1:1), polysorbate 80, sodium lauryl sulfate, triacetin, calcium stearate, talc
structure of a cover of gelatin capsules:
structure of a lid indigo carmine (E172), ferrous oxide red (E172), ferrous oxide yellow (E172), the titan dioxide (E171), gelatin
structure of the body - ferrous oxide red (E172), ferrous oxide yellow (E172), the titan dioxide (E171), gelatin
The description
Solid gelatin capsules of size No. 2 with the body of orange color and a lid of olive-green color, with marking 701 and the graphic representation of the trademark on the body and 0.4 on a lid.
Contents of capsules - granules of yellow-white or pale yellow color
Pharmacotherapeutic group
Drugs for treatment of urological diseases. Drugs for treatment of a benign hypertrophy of a prostate. Alpha adrenoblockers. Tamsulozin
ATX G04CA02 Code
Pharmacological
Pharmacokinetics Absorption properties:
Tamsulozin is soaked up in a small intestine, the bioavailability of drug is close to 100%. The concomitant use with food reduces bioavailability of a tamsulozin.
It is possible to provide constancy of conditions of absorption of drug, having recommended to the patient to accept tamsulozin after a breakfast or the first meal.
Pharmacokinetic indicators of a tamsulozin have linear character. After single dose of a tamsulozin on an empty stomach, peak plasma concentration of a tamsulozin is reached approximately in 6 hours, after achievement of an equilibrium state what requires about 5 days of multiple dose of drug, Cmax at patients is approximately two third higher, than is reached at single dose of drug. In spite of the fact that this pattern is noted at elderly people, it is expected that administration of drug by young people, will be followed by similar changes.
The significant individual distinctions are characteristic of plasma concentration of drug after single and multiple dose.
Distribution:
In an organism tamsulozin approximately for 99% contacts proteins of plasma.
Biotransformation:
Tamsulozin has low effect of the first passing. The most part of a tamsulozin is present at plasma in not changed look. Drug is metabolized in a liver.
Any of metabolites has no activity exceeding activity of the most active ingredient of drug. At insignificant and moderate degree of a liver failure the correction of the mode of dosing is not required.
Removal:
Tamsulozin and his metabolites are mainly allocated with urine, at the same time the share of not changed drug makes about 9%. It was established that after single dose of a tamsulozin after meal, and after achievement of an equilibrium state, time of semi-removal of drug is about 10 and 13 hours, respectively.
The volume of distribution of drug is about 0.2 l/kg.
The pharmacodynamics
Tamsulozin active ingredient of the drug Omnik is a selection competitive inhibitor of alfa1-adrenoceptors, drug has affinity to alfa1a and alfa1d to subtypes, linking of a tamsulozin with these receptors relaxes unstriated muscles of a prostate and an urethra.
Omnik increases the maximum flow rate of urine. Reduces obstruction by relaxation of smooth muscles in a prostate and an urethra, thereby facilitating urination disturbance symptoms.
Besides, drug reduces ischuria symptoms in which an important role is played by instability of a bladder.
These effects concerning symptoms of an ischuria and disturbance of urination remain at long therapy. Performing surgical treatment or catheterization is considerably deferred.
Antagonists of 1 adrenoceptors can reduce arterial blood pressure by reduction of peripheric vascular resistance. In the researches Omnika clinically significant lowering of arterial pressure is noted. At a tamsulozin genotoksichesky properties were not revealed.
Indications
- treatment of dysuric disorders in the benign hyperplasia of a prostate (BHP)
the Route of administration and doses
Inside, on 1 capsule after a breakfast. To accept capsules entirely, washing down with water. The capsule is not recommended to be chewed as it can affect drug release speed.
The course of treatment is defined individually by the doctor.
Side effects
Often (& gt, 1/100, & lt, 1/10)
- dizziness
- disturbances of an ejaculation
Infrequently (& gt, 1/1,000, & lt, 1/100)
- a headache
- tachycardia
- orthostatic hypotension
- rhinitis
- nausea, vomiting, a constipation, diarrhea
- rash, a skin itching, urticaria
- an asthenia
Seldom (& gt, 1/10,000, & lt, 1/1,000)
- a syncope
- a Quincke's disease
Very seldom (& lt, 1/10,000) - Stephens-Johnson's Syndrome - a priapism during operation for a cataract was noted development of a syndrome of intraoperative instability of an iris of the eye of an eye (syndrome of a narrow pupil) that was noted in post-marketing observations.
The contraindication
- hypersensitivity to active component of drug or to excipients
- orthostatic hypotension (including in the anamnesis) - the profound liver failure
- with care a heavy renal failure (clearance of creatinine & lt, 10 ml/min.)
- children's and teenage age up to 18 years
Medicinal interactions
was Not noted adverse medicinal interactions when assigning a tamsulozin along with atenolol, enalapril or theophylline.
The concomitant use of drug with Cimetidinum leads to increase in concentration of a tamsulozin in blood plasma, and reception with furosemide - to decrease in plasma concentration of drug, however, concentration remain within acceptable level.
In the researches in vitro with use of microsomes of a liver (reflect the processes happening to participation of isoforms of P450 cytochrome) the interaction at the level of hepatic metabolism with amitriptyline, salbutamol, glibenclamide and finasteridy was not revealed. However, diclofenac and warfarin can increase the clearance rate of a tamsulozin.
At co-administration with the drugs reducing arterial blood pressure, for example, drugs for the general anesthesia or other antagonists of 1 adrenoceptors, increase in their hypotensive action can be noted.
Simultaneous use of a tamsulozin with CYP3A4 inhibitors can lead to strengthening of effect of a tamsulozin. Co-administration of a ketokonazol (known strong CYP3A4 inhibitor) led to increase in AUC and Cmax of a tamsulozin respectively in 2.8 and 2.2 times.
Tamsulozin should not be applied in a combination with the expressed CYP3A4 inhibitors at patients of slow metabolizator with a phenotype of CYP2D6.
Tamsulozin it is necessary to use with care in a combination with the expressed and moderate CYP3A4 inhibitors.
Co-administration of a tamsulozin with paroksetiny, the expressed CYP2D6 inhibitor, was followed increase in Cmax and AUC of a tamsulozin respectively in 1.3 and 1.6 times, however these changes are regarded as clinically not significant.
Special instructions
As well as when using other antagonists of 1 adrenoceptors, in some cases treatment by Omnik can be followed by a lowering of arterial pressure, as a result, in rare instances perhaps development of an unconscious state. At the first symptoms of orthostatic hypotonia (dizziness, weakness) the patient should pass into a sitting position or lying before disappearance of symptoms.
Before an initiation of treatment Omnik it is necessary to perform examination of the patient for an exception of other states, the followed similar symptoms with a benign hyperplasia of a prostate. Before an initiation of treatment and subsequently through regular intervals it is necessary to conduct a manual rectal research and if it is necessary, definition of prostatspetsifichesky antigen (DOG).
Treatment of patients with a heavy renal failure (clearance of creatinine & lt, 10 ml/min.) has to be performed with care as researches at such patients were not conducted.
At the certain patients who were receiving or earlier accepting tamsulozin at operational treatment concerning a cataract or glaucoma the intraoperative syndrome of a flabby iris (Intraoperative Floppy Iris Syndrome, IFIS), option of a syndrome of a narrow pupil was observed. Existence of IFIS can increase risk of complications from eyes in time and after operation.
From experience it is known that there can be useful a cancellation of a tamsulozin of a hydrochloride in 1-2 weeks prior to operation, but effects of such cancellation of therapy are not established yet. Also it was reported about development of IFIS in the patients who stopped reception of a tamsulozin long before operation.
At patients to whom carrying out operational treatment of a cataract or glaucoma is planned, are not recommended to begin therapy of a tamsulozin with a hydrochloride. In process of a preparation for surgery the surgeons and ophthalmologists have to specify, accepts or whether the patient to whom operational treatment of a cataract or glaucoma is planned, tamsulozin accepted earlier to be ready to treatment of IFIS in case of its development during operation.
Pregnancy and the period of a lactation
Drug is intended only for treatment of men. Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms of the Research of influence on ability to driving of the car and to control of mechanisms were not carried out. However, patients have to show care, in connection with a possibility of development of dizziness.
Overdose
Symptoms: heavy arterial hypotension. Treatment: administration of drugs with angiotonic and cardiotonic action is necessary. Transfer of the patient to horizontal position can restore normal arterial blood pressure and heart rate. At inefficiency of these actions carrying out the measures directed to increase in volume of the circulating blood and also introduction of vazopressor is possible. Are necessary assessment of function of kidneys in dynamics and performing maintenance therapy. Because Omnik actively contacts proteins - the hemodialysis is probably inefficient. For treatment of poisonings with high doses of drug performing gastric lavage, intake of activated carbon or osmotic laxatives, such as sodium sulfate is reasonable.
The form of release and packing
On 10 capsules place in blister strip packaging from a film of polyvinylchloride and printing aluminum foil.
On 1 or 3 blister strip packagings together with the instruction for medical use in the state and Russian languages place in a pack from cardboard.
To Store storage conditions at a temperature of 15 - 25 0C.
To store out of children's reach!
4 years
not to apply a period of storage after expiry date.
Prescription status
According to the prescription
the Name and the country
of the Astellas Pharma manufacturing organization Europe B.V., Silviusveg 62, 2333 VE, Leiden, the Netherlands
the Name and the country of the owner of the registration certificate
Astellas of Pharm B.V. Europe, Silviusveg 62, 2333 VE, Leiden, Netherlands
The address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods):
Representation Astellas of Pharm B.V. Europe in RK
Almaty, 050559, Al-Farabi Ave. 15, Nurla Tau's Centre Party of Finland, zd 4B,
office No. 20 Phone number (727) 311-13-89 Fax (727) 311-13-90
www.astellas.ru,
To develop e-mail of astellas@com.kz