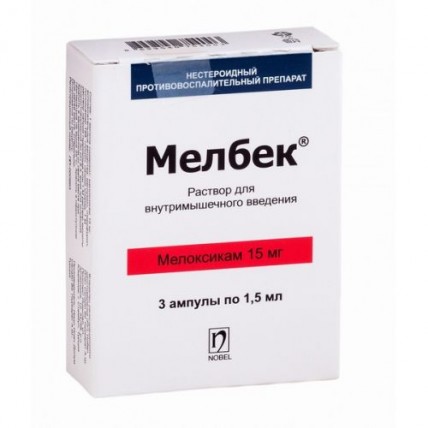
Melbek 15 mg / 1.5 ml injection 3's / m
- $14.00
The instruction for medical use of MELBEK® medicine Trade name МЕЛБЕК® the International unlicensed name Meloksikam Lekarstvennaya a form Solution for intramuscular injections, 15mg/1.5 ml One ampoule of 1.5 ml contains Structure: active agent - to meloksika of 15 mg, excipients: meglumin, glikofurol, half-oxameasures 188, glycine, sodium chloride, sodium hydroxide of 1 M solution or acid of chlorohydrogen 1 M, water for injections. Description Transparent solution of yellow color Pharmacotherapeutic group Anti-inflammatory and antirheumatic drugs. Non-steroidal anti-inflammatory drugs. Oksikama. Meloksikam. The ATX M01AC06 code the Pharmacological Meloksikam Pharmacokinetics properties is completely absorbed after intramuscular introduction. The relative bioavailability in comparison with bioavailability at intake is nearly 100%. Therefore upon transition from a dose, injection on oral forms of selection, it is not required. After introduction of 15 mg of drug intramusculary the peak concentration in plasma making 1.6-1.8 mkg/ml is reached in 60-96 min. Distribution. Meloksikam intensively contacts proteins of plasma, especially albumine (99%). Gets into synovial fluid where concentration makes him about 50% of concentration in plasma. Distribution volume low, averages 11 l, individual distinctions are 7 – 20%. Biotransformation. Meloksikam is exposed to considerable biotransformation in a liver. In urine four various metabolites inactive in the pharmakodinamichesky relation are defined. The main metabolite (5 '-karboksimeloksikam (60% of dose size)) is formed by oxidation of an intermediate metabolite (5 '-hydroksimetilmeloksikama (9% of dose size)). Peroxidase takes part in formation of two other metabolites making 16% and 4% of drug dose size probably. Removal. Meloksikam is brought equally through intestines and kidneys, in not changed look – 5% of a daily dose through intestines, in urine in not changed look drug is found only in trace quantities. Elimination half-life varies from 13 to 25 hours after introduction. The general plasma clearance is 7-12 ml/min. Linearity/nonlinearity of pharmacokinetics. Meloksikam shows linear pharmacokinetics in doses of 7.5 mg - 15 mg at intake or intramuscular introduction. Pharmacokinetics at special groups of patients the Renal/liver failure the Liver failure and also a renal failure from easy to moderate severity of significant effect on pharmacokinetics of a meloksikam do not render. In a terminal renal failure the increase in volume of distribution can lead to increase in concentration of a free meloksikam. Elderly people Elderly men have the pharmacokinetic parameters similar to parameters of young men. Elderly women have higher AUC values and longer elimination half-life in comparison with patients of young age. Pharmacodynamics МЕЛБЕК® non-steroidal anti-inflammatory drug (NPVP) from a class of enoliyevy acid, the having anti-inflammatory, soothing and febrifugal effect. The mechanism of above-mentioned effects consists in ability of the drug MELBEK® to inhibit biosynthesis of prostaglandins which are inflammation mediators. The mechanism of action is connected with mainly selection inhibition of cyclooxygenase-2 (TsOG-2) - the specific enzyme participating in development of processes of inflammation. It is considered that the inhibition of TsOG-2 provides therapeutic effect of NPVP whereas the inhibition of constantly present isoenzyme of TsOG-1 can be the cause of side effects from a stomach and kidneys. Indications to primeneniyukratkosrochny symptomatic treatment of acute exacerbations of a pseudorheumatism and ankylosing spondylarthritis when the oral way or a rectal way of use is unacceptable. Route of administration and doses Intramuscular administration of the drug MELBEK® is shown only during the first several days of treatment, but no more than 2-3 days (at unacceptability of oral or rectal administration of drug). Probability of undesirable reactions increases together with a dose and duration of influence therefore it is necessary to use the smallest possible duration and the minimum effective daily dose. The general daily dose of the drug MELBEK® should be accepted once. The maximum recommended daily dose of the drug MELBEK® irrespective of a form of release makes 15 mg. A pain syndrome in an osteoarthritis: 7.5 mg/days. If necessary it is possible to increase a dose to 15 mg/days. Pseudorheumatism: 15 mg/days. According to therapeutic reaction it is possible to reduce a dose to 7.5 mg/days. Ankylosing spondylarthritis: 15 mg/days. According to therapeutic reaction it is possible to reduce a dose to 7.5 mg/days. Special groups of patients. At patients of advanced age and patients with the increased risk of development of undesirable reactions, for example, gastrointestinal diseases or risk factors for cardiovascular diseases, treatment it is necessary to begin 7.5 mg/days in a dose. At insignificant or moderate depression of function of kidneys (the clearance of creatinine is reduced no more than by 25 ml/min. from norm) the dose decline is not required. To the patients with heavy renal failures who are not on a hemodialysis, the drug MELBEK® is contraindicated. The maximum daily dose of the drug MELBEK® in ampoules at the patients with a heavy renal failure who are on a hemodialysis should not exceed 7.5 mg. The initial dose at patients with the increased risk of side effects makes 7.5 mg/days. Administration of MELBEK® solution is carried out by deep intramuscular injections. Intravenous administration of MELBEK® solution is forbidden. Considering possible incompatibility, contents of ampoules should not be mixed in one syringe with other medicines! Side effects Use of some NPVS (especially at high doses and at long-term treatment) can be connected with small increase in risk of arterial trombotichesky complications (for example, a myocardial infarction or a stroke). At use of NPVS such phenomena as hypostases, increase in arterial blood pressure and heart failure, in connection with treatment were registered. The most often observed side effects are disturbances of bodies of digestive tract: round ulcers, perforation or gastrointestinal bleedings, sometimes from the death, especially at elderly people, nausea, vomiting, diarrhea, a meteorism, a constipation, dyspepsia, an abdominal pain, a melena, a stomacace, exacerbation of colitis and Crohn's disease. Gastritis is less often observed. Also the expressed skin side reactions (SCAR) were registered: Stephens-Johnson's syndrome (SJS) and toxic epidermal necrolysis (TEN). The undesirable phenomena are given below with use of the following classification: very often ≥ 1/10, it is frequent ≥1/100 to & lt, 1/10, infrequently ≥1/1000 to & lt, 1/100, is rare ≥1/10000 to & lt, 1/1000, also lt, 10000 is very rare, it is not known – cannot be defined from available data. Disturbances of blood and lymphatic systems Infrequently: anemia Seldom: change of a blood count (including change of a leukocytic formula), a leukopenia, thrombocytopenia, a cytopenia (at simultaneous use of potentially myelotoxic medicines, in particular a methotrexate) Disturbances of the immune system Infrequently: other reactions of immediate hypersensitivity it is rare: acute anaphylaxis, anaphylactic reactions, anaphylactoid reactions Mental disturbances Seldom: change of mood Is unknown: confusion of consciousness, disturbance of orientation of Disturbance of nervous system It is frequent: a headache It is unknown: dizziness, drowsiness Ophthalmologic disturbances Seldom: conjunctivitis, disorder of vision, including. illegibility of sight of Narusheniya of organs of hearing Infrequently: dizziness Is rare: sonitus Warm disturbances Seldom: heartbeat Vascular disorders Infrequently: increase in arterial blood pressure, feeling of rush of blood to the person Narusheniya of respiratory organs Is rare: acute development of bronchial asthma (at patients with an allergy to acetylsalicylic acid or other NPVP) Gastrointestinal disturbances Often: abdominal pain, dyspepsia, diarrhea, nausea, vomiting Infrequently: concealed or obvious gastrointestinal hemorrhage, gastritis, constipation, meteorism, eructation, stomatitis Seldom: gastroduodenal ulcer, colitis, esophagitis Very seldom: perforation of digestive tract (the lethal outcome is possible) Narusheniya of a gepatobiliarny system Infrequently: passing changes of indicators of function of a liver (for example, increase in activity of transaminases or bilirubin) It is very rare: hepatitis Skin disturbances Infrequently: itching, angioedema Seldom: toxic epidermal necrolysis, Stephens-Johnson's syndrome, small tortoiseshell Very seldom: bullous dermatitis, a multiformny erythema, rash It is unknown: Narusheniya's photosensitization of an urinogenital system Infrequently: changes of indicators of function of kidneys (increase in level of creatinine and/or urea in blood serum) It is very rare: an acute renal failure, difficulty at urination, a sharp ischuria of Narusheniya from a reproductive system: It is unknown: female infertility, ovulation delay General disturbances and local reactions Often: puffiness in the injection site, painful feelings in the injection site Infrequently: Contraindication hypostases - hypersensitivity to a meloksikam, acetylsalicylic acid and other NPVS - bronchial asthma, polyposes mucous a nose, the Quincke's disease or urtikarny rashes caused by intake of acetylsalicylic acid or other NPVS - a peptic ulcer of a stomach and duodenum in a phase obostre-a niya, nonspecific ulcer colitis and Crohn's disease - gastrointestinal bleeding, cerebrovascular bleeding or other hemorrhagic diseases - a heavy liver and renal failure - the profound uncontrollable (dekompensirovanny) heart failure - a pain syndrome after operation of coronary shunting (imposing of a roundabout anastomosis) - pregnancy and the period of a lactation - the children's and teenage age up to 18 years Medicinal interactions At co-administration МЕЛБЕК® with salicylates and other non-steroidal anti-inflammatory drugs increases risk of development of ulcer damages and bleedings from digestive tract. At combined use МЕЛБЕК® and anticoagulants, the tiklopidina, heparin, warfarin, trombolitik raises risk of developing gastrointestinal bleedings. It is not necessary to appoint to the patients receiving anticoagulants. МЕЛБЕК® can lead to increase in concentration of lithium in blood at use with lithium-containing drugs. At joint appointment with a methotrexate and other potentially miyelotoksichny drugs the risk of development of a pancytopenia increases. In such cases the monitoring quantity of blood cells is necessary. Simultaneous use МЕЛБЕК® and diuretics can lead to development of an acute renal failure in patients with symptoms of dehydration of an organism. It is necessary to compensate dehydration and to watch function of kidneys. МЕЛБЕК® weakens effect of β-adrenoblockers, APF inhibitors, vazodilatator and diuretics. Holestiramin considerably increases clearance of a meloksikam. Simultaneous use with cyclosporine can lead to nephrotoxicity strengthening as МЕЛБЕК® reduces synthesis of prostaglandins synthesized in kidneys. NPVS can cause a delay of sodium, potassium, liquid and to weaken effect of saluretics. As a result at predisposed patients the purpose of NPVS can lead to progressing of heart failure and arterial hypertension the Combined use of 200 mg of Cimetidinum does not influence pharmacokinetics of a single dose of a meloksikam. Digoxin has no considerable impact on pharmacokinetics of a meloksikam. It is impossible to exclude a possibility of medicinal interaction of a meloksikam with hypoglycemic means. Antihypertensive drugs (for example, beta-blockers, APF inhibitors) – decrease in efficiency of antihypertensive drugs – vazodilatator was observed. Meloksikam can is mediated through renal prostaglandins to increase insufficiency of cyclosporines. The special instructions MELBEK® in ampoules it is not intended for intravenous administration! It is not necessary to exceed the recommended maximum daily dose in case of insufficient therapeutic effect and also it is not necessary to use NPVP as additional therapy as it can lead to increase in toxicity at unproven therapeutic advantages of a combination. It is necessary to avoid combined use of a meloksikam and other NPVP, including selective inhibitors of cyclooxygenase-2. Meloksikam is not suitable for treatment of the patients needing removal of acute painful symptoms. If within several days the improvement is not observed, it is necessary to estimate clinical advantage of treatment anew. It is necessary to study attentively any cases of an esophagitis, gastritis and/or round ulcer in the anamnesis to make sure of an absolute recovery of the patient prior to treatment meloksikamy. It is regularly necessary to control a condition of the patient with similar diseases in the anamnesis receiving to meloksika regarding a possible recurrence. Influence on digestive system As well as at use of other NPVP, at treatment can arise potentially fatal gastrointestinal bleeding, an ulceration or perforation as with existence of alarming symptoms or serious gastrointestinal violations in the anamnesis at any time, and without them. The risk of gastrointestinal bleeding, an ulceration or perforation increases in direct ratio to NPVP doses at patients with a peptic ulcer in the anamnesis, especially at its complication bleeding or perforation and at patients of advanced age. Such patients have to begin treatment with the smallest possible dose. It is necessary to consider the possibility of purpose of combination therapy with drugs of protective action (for example, a mizoprostol or inhibitors of the proton pump) to these patients and also the patients needing intake of acetylsalicylic acid in low doses or other drugs increasing risk of emergence of the phenomena from digestive tract. It is necessary to show care at treatment of patients with a gastrointestinal disease in the anamnesis. It is necessary to control patients with gastrointestinal symptoms. The combination with meloksikamy is not recommended to the patients receiving the accompanying drugs capable to increase risk of an ulceration or bleeding, for example heparin as remedy or among general purposes to patients of advanced age, other non-steroidal anti-inflammatory drugs or acetylsalicylic acid in doses ≥ 500 mg once or at the general daily dose ≥ 3 g. МЕЛБЕК® it is necessary to cancel when developing a round ulcer or gastrointestinal bleeding. NPVP needs to be applied with care to patients with gastrointestinal diseases in the anamnesis (ulcer colitis, Crohn's disease) because of possible exacerbation of these diseases. The cardiovascular and cerebrovascular phenomena from easy to moderate severity in the anamnesis are necessary for Patients with hypertensia and/or stagnant heart failure appropriate monitoring and medical consultations as at therapy of NPVP the delay of liquid and hypostases were noted. Clinical control of arterial blood pressure at the initial level and especially at the initial stage of treatment meloksikamy is recommended to the patients who are in risk group. Use of some NPVP, including a meloksikama (especially in high doses at long-term treatment), can be associated with small increase in risk of emergence of the arterial trombotichesky phenomena (for example, a myocardial infarction or a stroke). The data allowing to exclude such risk for a meloksikam are insufficient. Patients with uncontrollable hypertensia, the stagnant heart failure diagnosed by coronary heart disease, a disease of peripheral arteries and/or a cerebrovascular disease can carry out treatment only the meloksikamy ambassador of careful estimates of its expediency. The same assessment should be carried out prior to long-term treatment of patients with risk factors of developing a cardiovascular disease (among them hypertensia, a lipidemia, diabetes, smoking). The skin reactions Menacing zhi
skin reactions, for example the Stephens's syndrome — Johnson (SSJ) and also the toxic epidermal necrolysis (TEN) were noted at reception of a meloksikam. The greatest risk of these reactions at patients, apparently, takes place at the beginning of therapy, and in most cases reaction is shown within the first month of treatment. Administration of drug МЕЛБЕК® should be stopped at the first appearance of skin rash, defeats mucous or any other sign of hypersensitivity. The best results at treatment of SSD and TEN can achieve at early diagnosing and the immediate termination of reception of any suspect of drug. The early termination of therapy is associated with the best forecast. If at the patient as a result of reception of a meloksikam SSD or TEN developed, reception of a meloksikam should not be resumed at this category of patients. Indicators of function of a liver and kidneys As well as at use of other NPVP, increase in level of transaminases in blood serum, increase in bilirubin in serum or other parameters of function of a liver and also increase in level of creatinine in serum and urea nitrogen of blood and other deviations of laboratory indicators is sometimes noted. In most cases these effects represented the slight taking place increase of parameters above normal values. If such anomaly is considerable or resistant, administration of drug МЕЛБЕК® it is necessary to stop and perform necessary examination of the patient with the subsequent observation. For patients with clinically stable cirrhosis the dose decline is not required. The functional renal failure of NPVP, synthesis of prostaglandins of kidneys which play a supporting role in maintenance of a blood-groove in kidneys can cause a functional renal failure, reducing glomerular filtration rate. This undesirable phenomenon is dose-dependent. In an initiation of treatment or after increase in a dose it is recommended to check carefully function of kidneys, including diuresis volume at patients with the following risk factors: - patients of advanced age, - the accompanying treatment, for example APF inhibitors, to antagonists of angiotensin-II, a sartana, diuretics, - a hypovolemia (irrespective of the reason), - stagnant heart failure, - a renal failure, - a nephrotic syndrome, - a lupoid nephropathy, - a heavy abnormal liver function (seralbumin & lt, 25 g/l or assessment ≥ 10 on Chayld's scale — I Drink). In rare instances NPVP can cause interstitial nephrite, a glomerulonephritis, medullary necrosis of kidneys or a nephrotic syndrome. The drug MELBEK® dose at the patients with an end-stage of a renal failure who are on a hemodialysis should not exceed 7.5 mg. For patients with slight and moderate renal failures (i.e. patients with clearance of creatinine have more than 25 ml/min.) the dose decline is not required. NPVP can cause a delay of sodium, potassium and water and to prevent natriuretic effect of diuretics. As a result at the patients subject to these effects, heart failure or hypertensia can amplify or become aggravated. For patients of risk group the clinical monitoring is recommended. The hyperpotassemia can contribute to the Development of a hyperpotassemia diabetes or the accompanying treatment which increases potassium content in blood. In these cases it is necessary to trace potassium level regularly. Combined use with pemetreksedy Patients with a renal failure from easy to moderate severity need to interrupt reception of a meloksikam at least in 5 days prior to introduction of a pemetreksed, not to accept to meloksika in day of introduction and within not less than 2 days after introduction of a pemetreksed. Other preventions and precautionary measures the Painful or weakened patients can worse transfer side effects, in this case careful control is necessary. As well as at use of other NPVP, it is necessary to show care at treatment of elderly patients who more likely suffer from a renal failure, a liver or heart. At patients of advanced age higher frequency of emergence of undesirable reactions to NPVP, especially gastrointestinal bleeding and perforation which can lead to a lethal outcome is observed. Meloksikam, as well as any other NPVP, can mask symptoms of the basic infectious disease. As well as at reception of other NPVP entered in oil in the place of an injection, abscesses can be formed and develop necrosis. This medicine contains less than 1 mmol of sodium (23 mg) on an ampoule of 1.5 ml, i.e. practically "does not contain sodium". Fertility, pregnancy and period of a lactation Fertility. Use of a meloksikam, as well as any drug inhibiting cyclooxygenase/synthesis of prostaglandins can affect reproductive ability and is not recommended to the women planning pregnancy. Thus, for women who have difficulties with conception or are exposed to researches in connection with infertility, it is necessary to consider cancellation of reception of a meloksikam. Pregnancy. The drug MELBEK® is contraindicated during pregnancy. The inhibition of synthesis of prostaglandins can negatively affect pregnancy and/or development of an embryo and fruit. Data of epidemiological researches indicate the increased risk of an abortion, heart diseases and a gastroshizis after intake of inhibitors of synthesis of prostaglandins in the early stages of pregnancy. During the third trimester of pregnancy all inhibitors of synthesis of prostaglandins can subject a fruit: - to cardiopulmonary toxicity (with premature closing of a botallov of a channel and pulmonary hypertensia) - to a renal failure which can progress to a renal failure with an oligoamnios, mother and a fruit at the end of pregnancy: - to possible increase in a bleeding time, effect of anti-aggregation which can arise even at very low doses - to the suppression of reductions of a uterus leading to a delay or increase in a duration of delivery. Lactation period. It is known that NPVP get into breast milk. Though special researches of the drug MELBEK® in this regard were not conducted, it is necessary to avoid its use for the women nursing. Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms of the Research of influence on ability to drive the car and to use mechanisms were not carried out. Nevertheless, patients should be informed on possible manifestation of undesirable effects, such as disorder of vision, including illegibility of sight, dizziness, drowsiness, other disturbances of the central nervous system. In case of any of the specified side effects the patients have to avoid control of vehicles and refuse work with potentially dangerous mechanisms. Overdose Symptoms: slackness, block, drowsiness, nausea, vomiting, pain in epigastric area, gastrointestinal bleeding, anaphylactoid reactions, symptoms of a serious poisoning: increase in arterial blood pressure, acute renal failure, abnormal liver function, respiratory depression, coma, convulsions, vascular collapse and cardiac arrest. Treatment: there is no specific antidote, the symptomatic and supporting treatment. The artificial diuresis, urine alkalization, a hemodialysis or hemoperfusion are ineffective (at a meloksikam high extent of linking with proteins of blood plasma). Form of release and upakovkapo 1.5 ml of drug place in an ampoule from colourless transparent glass with the line for a break. Apply the text on an ampoule with method of an intaglio printing the fast fixed paint. On 3 ampoules in the plastic dividing separator together with the instruction for use in the state and Russian languages place in a pack from cardboard. To Store storage conditions at a temperature not over 25 of 0C, the place protected from light. To store out of children's reach! A period of storage 4 years not to apply after a period of storage Prescription status According to the prescription the Producer/packer "An idol Ilach Dolum Sanai ve A.Sh. Tidzharet", Istanbul, Turkey the Owner of the registration certificate of JSC Nobel Almatinskaya Pharmatsevticheskaya Fabrika Republic of Kazakhstan Almaty, Shevchenko St. 162 E. The address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of medicines and responsible for post-registration observation of safety of medicine: JSC Nobel Almatinskaya Pharmatsevticheskaya Fabrika Republic of Kazakhstan, Almaty, Shevchenko St. 162 E.
To develop
skin reactions, for example the Stephens's syndrome — Johnson (SSJ) and also the toxic epidermal necrolysis (TEN) were noted at reception of a meloksikam. The greatest risk of these reactions at patients, apparently, takes place at the beginning of therapy, and in most cases reaction is shown within the first month of treatment. Administration of drug МЕЛБЕК® should be stopped at the first appearance of skin rash, defeats mucous or any other sign of hypersensitivity. The best results at treatment of SSD and TEN can achieve at early diagnosing and the immediate termination of reception of any suspect of drug. The early termination of therapy is associated with the best forecast. If at the patient as a result of reception of a meloksikam SSD or TEN developed, reception of a meloksikam should not be resumed at this category of patients. Indicators of function of a liver and kidneys As well as at use of other NPVP, increase in level of transaminases in blood serum, increase in bilirubin in serum or other parameters of function of a liver and also increase in level of creatinine in serum and urea nitrogen of blood and other deviations of laboratory indicators is sometimes noted. In most cases these effects represented the slight taking place increase of parameters above normal values. If such anomaly is considerable or resistant, administration of drug МЕЛБЕК® it is necessary to stop and perform necessary examination of the patient with the subsequent observation. For patients with clinically stable cirrhosis the dose decline is not required. The functional renal failure of NPVP, synthesis of prostaglandins of kidneys which play a supporting role in maintenance of a blood-groove in kidneys can cause a functional renal failure, reducing glomerular filtration rate. This undesirable phenomenon is dose-dependent. In an initiation of treatment or after increase in a dose it is recommended to check carefully function of kidneys, including diuresis volume at patients with the following risk factors: - patients of advanced age, - the accompanying treatment, for example APF inhibitors, to antagonists of angiotensin-II, a sartana, diuretics, - a hypovolemia (irrespective of the reason), - stagnant heart failure, - a renal failure, - a nephrotic syndrome, - a lupoid nephropathy, - a heavy abnormal liver function (seralbumin & lt, 25 g/l or assessment ≥ 10 on Chayld's scale — I Drink). In rare instances NPVP can cause interstitial nephrite, a glomerulonephritis, medullary necrosis of kidneys or a nephrotic syndrome. The drug MELBEK® dose at the patients with an end-stage of a renal failure who are on a hemodialysis should not exceed 7.5 mg. For patients with slight and moderate renal failures (i.e. patients with clearance of creatinine have more than 25 ml/min.) the dose decline is not required. NPVP can cause a delay of sodium, potassium and water and to prevent natriuretic effect of diuretics. As a result at the patients subject to these effects, heart failure or hypertensia can amplify or become aggravated. For patients of risk group the clinical monitoring is recommended. The hyperpotassemia can contribute to the Development of a hyperpotassemia diabetes or the accompanying treatment which increases potassium content in blood. In these cases it is necessary to trace potassium level regularly. Combined use with pemetreksedy Patients with a renal failure from easy to moderate severity need to interrupt reception of a meloksikam at least in 5 days prior to introduction of a pemetreksed, not to accept to meloksika in day of introduction and within not less than 2 days after introduction of a pemetreksed. Other preventions and precautionary measures the Painful or weakened patients can worse transfer side effects, in this case careful control is necessary. As well as at use of other NPVP, it is necessary to show care at treatment of elderly patients who more likely suffer from a renal failure, a liver or heart. At patients of advanced age higher frequency of emergence of undesirable reactions to NPVP, especially gastrointestinal bleeding and perforation which can lead to a lethal outcome is observed. Meloksikam, as well as any other NPVP, can mask symptoms of the basic infectious disease. As well as at reception of other NPVP entered in oil in the place of an injection, abscesses can be formed and develop necrosis. This medicine contains less than 1 mmol of sodium (23 mg) on an ampoule of 1.5 ml, i.e. practically "does not contain sodium". Fertility, pregnancy and period of a lactation Fertility. Use of a meloksikam, as well as any drug inhibiting cyclooxygenase/synthesis of prostaglandins can affect reproductive ability and is not recommended to the women planning pregnancy. Thus, for women who have difficulties with conception or are exposed to researches in connection with infertility, it is necessary to consider cancellation of reception of a meloksikam. Pregnancy. The drug MELBEK® is contraindicated during pregnancy. The inhibition of synthesis of prostaglandins can negatively affect pregnancy and/or development of an embryo and fruit. Data of epidemiological researches indicate the increased risk of an abortion, heart diseases and a gastroshizis after intake of inhibitors of synthesis of prostaglandins in the early stages of pregnancy. During the third trimester of pregnancy all inhibitors of synthesis of prostaglandins can subject a fruit: - to cardiopulmonary toxicity (with premature closing of a botallov of a channel and pulmonary hypertensia) - to a renal failure which can progress to a renal failure with an oligoamnios, mother and a fruit at the end of pregnancy: - to possible increase in a bleeding time, effect of anti-aggregation which can arise even at very low doses - to the suppression of reductions of a uterus leading to a delay or increase in a duration of delivery. Lactation period. It is known that NPVP get into breast milk. Though special researches of the drug MELBEK® in this regard were not conducted, it is necessary to avoid its use for the women nursing. Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms of the Research of influence on ability to drive the car and to use mechanisms were not carried out. Nevertheless, patients should be informed on possible manifestation of undesirable effects, such as disorder of vision, including illegibility of sight, dizziness, drowsiness, other disturbances of the central nervous system. In case of any of the specified side effects the patients have to avoid control of vehicles and refuse work with potentially dangerous mechanisms. Overdose Symptoms: slackness, block, drowsiness, nausea, vomiting, pain in epigastric area, gastrointestinal bleeding, anaphylactoid reactions, symptoms of a serious poisoning: increase in arterial blood pressure, acute renal failure, abnormal liver function, respiratory depression, coma, convulsions, vascular collapse and cardiac arrest. Treatment: there is no specific antidote, the symptomatic and supporting treatment. The artificial diuresis, urine alkalization, a hemodialysis or hemoperfusion are ineffective (at a meloksikam high extent of linking with proteins of blood plasma). Form of release and upakovkapo 1.5 ml of drug place in an ampoule from colourless transparent glass with the line for a break. Apply the text on an ampoule with method of an intaglio printing the fast fixed paint. On 3 ampoules in the plastic dividing separator together with the instruction for use in the state and Russian languages place in a pack from cardboard. To Store storage conditions at a temperature not over 25 of 0C, the place protected from light. To store out of children's reach! A period of storage 4 years not to apply after a period of storage Prescription status According to the prescription the Producer/packer "An idol Ilach Dolum Sanai ve A.Sh. Tidzharet", Istanbul, Turkey the Owner of the registration certificate of JSC Nobel Almatinskaya Pharmatsevticheskaya Fabrika Republic of Kazakhstan Almaty, Shevchenko St. 162 E. The address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of medicines and responsible for post-registration observation of safety of medicine: JSC Nobel Almatinskaya Pharmatsevticheskaya Fabrika Republic of Kazakhstan, Almaty, Shevchenko St. 162 E.
To develop