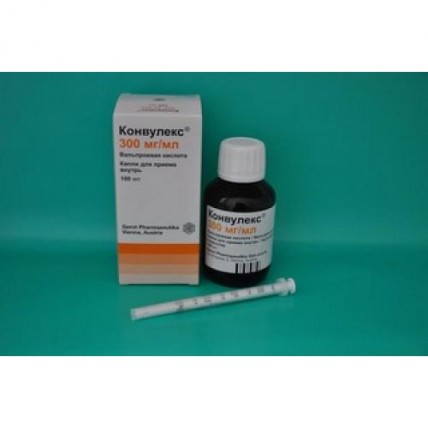
Konvuleks 300 mg / ml 100 ml drops for oral administration
- $25.00
The instruction for medical use
of Konvuleks® medicine
the Trade name
of Konvuleks®
the International unlicensed
name Valproic Acid Dosage Form
of the Drop oral
Structure
of 1 ml of solution contains
active agent - sodium Valproatum of 300 mg, (it is equivalent to valproic acid of 260.30 mg and sodium to hydroxide of 72.20 mg),
excipients: sodium saccharin, fragrance orange, sodium hydroxide, acid chlorohydrogen, the water purified
the Description
Transparent solution from colourless till slightly yellowish color with a sweet orange flavor and easy burning aftertaste.
Pharmacotherapeutic group
Antiepileptic drugs. Fatty acids derivatives. Valproic acid.
The ATX N03AG01 code
the Pharmacological
Pharmacokinetics Valproic Acid properties quickly and almost is completely soaked up in a GIT, the bioavailability at intake is 100%. Meal does not reduce absorption speed. The maximum level of concentration in plasma is noted in 3-4 h. Equilibrium concentration is reached for 2-4 day of treatment, depending on dosing intervals. Therapeutic concentration of drug in blood plasma fluctuates within 50-150 mg/l. Valproic acid is connected with proteins of plasma for 90-95% at concentration in blood plasma up to 50 mg/l and for 80-85% at concentration of 50-100 mg/l, in uraemia, a hypoproteinemia and cirrhosis the linking with proteins is reduced. Concentration levels in cerebrospinal fluid correlate with the size of the fraction of drug which is not connected with proteins. Valproic acid gets through a placental barrier, is emitted with breast milk. Concentration in breast milk makes 1-10% of concentration in blood plasma of mother. Drug is exposed to a glyukuronirovaniye and oxidation in a liver, metabolites and not changed valproic acid (1-3% of a dose) are removed by kidneys, small amounts are removed with excrements and with expired air. Elimination half-life of drug makes at healthy examinees and at monotherapy from 8 to 24 h, at a combination to other medicines the elimination half-life can make 6-8 h owing to induction of metabolic enzymes, at patients with an abnormal liver function and elderly patients can be much longer.
The prolonged form is characterized by lack of latent time of absorption, slow absorption, lower (for 25%), but rather more stable concentration in plasma between 4 and 14 h.
The pharmacodynamics
Convulexum – antiepileptic means, also has the central myorelaxation and sedative effect. The mechanism of action is caused mainly by inhibition of enzyme of GAMK-transferase and increase in content of piperidic acid (GAMK) in the central nervous system (CNS). GAMK interferes pre- and to postsynaptic categories and, thereby, prevents distribution of convulsive activity to central nervous system. Besides, in the mechanism of effect of drug the essential role belongs to impact of valproic acid on GAMK A receptors and also influence on a voltage - dependent Na-channels. Affects sites of postsynaptic receptors, imitating or enhancing the braking effect of GAMK. Potential direct impact on activity of membranes is connected with changes in conductivity of potassium. Improves a mental condition and mood of patients, has antiarrhytmic activity.
Indications
- epileptic seizures (including generalized and partial and also against the background of organic diseases of a brain)
- prevention of attacks of migraine
- the manic-depressive syndrome with a bipolar course when lithium is contraindicated or is not transferred by the patient
the Route of administration and doses
take the Drug inside, 2-3 times a day, in time or after a meal, with a small amount of liquid.
Adults. An initial dose at monotherapy - 5-15 mg/kg/days, at combination therapy – 10-30 mg/kg/days then gradually increase this dose by 5-10 mg/kg/week.
An average daily dose - 20-30 mg/kg of body weight.
The daily dose can be increased up to 60 mg/kg at a possibility of the organization of control of concentration of drug in blood plasma.
Children to the 6th summer age. An average daily dose at monotherapy – 15-45 mg/kg, maximum – 50mg/kg. At combination therapy of-30-100 mg/kg/days.
The dosage at children of 6 years is also more senior makes 5-15 mg/kg with gradual increase up to 20-30 mg/kg a day. At children who need a dose higher than 40 mg/kg a day biochemical and hematologic parameters have to be traced.
Average daily doses:
Age
Body weight
the Average dose
(in kg)
(mg/day)
of ml/day
of 3 - 6 months
5.5 -
7.5 150 0.5 ml
of 6 - 12 months
7.5 - 10,150 -
300 0.5 - 1 ml
of 1 - 3 years
10 - 15
300 - 450 1 - 1.5 ml
of 3 - 6 years
15 -
20 450 - 600 1.5 - 2 ml
of 7 - 11 years
of
20 - 40 600 - 1200 2 - 4 ml
of 12 - 17 years
40 - 60 1000 - 1500
ca. 3.5 - 5 ml
Adult (including elderly)
³
60 1200 - 2100 4 - 7 ml
Advanced age. Though the pharmacokinetics of Valproatums at advanced age has the features, it has limited clinical value, and the dose should be determined by clinical effect. Owing to reduction of linking with seralbumin the share of untied drug in plasma increases. It causes expediency of more careful selection of a dose of drug at elderly, with possible use of smaller doses of drug.
Patients with a renal failure. It can be necessary to lower a drug dose. The dose should be selected for monitoring of a clinical state as concentration indicators in plasma can be insufficiently informative.
Use of the portioning device.
1. To lower the piston in the syringe down, against the stop, then to place the syringe in a glass small bottle.
2. To lift the piston up until the mark in the piston corresponds to the ordered dosage (graduation in ml and mg). If necessary to repeat process before achievement of the general appointed quantity.
3. By means of pressing of the piston down, apply the measured dose in a small amount of liquid.
4. After each use, close a small bottle, and carefully rinse the syringe with water. You store both the syringe and a small bottle in a cardboard box.
Side effects
Side effects are possible generally at drug level in plasma higher than 100 mg/l or at the combined therapy.
Often
- nausea, vomiting, anorexia or increase in appetite, diarrhea, gastralgias, hepatitis
- a tremor
- a diplopia, flashing of 'front sights' before eyes
- anemia, thrombocytopenia, the decrease in content of fibrinogen, aggregation of thrombocytes and blood clotting which is followed by lengthening of a bleeding time, petekhialny hemorrhages, bruises, hematomas, bleeding, an agranulocytosis, a lymphocytosis
- decrease or increase in body weight
- a giperkreatininemiya, a giperammoniyemiya, a hyperbilirubinemia, insignificant increase in activity of 'hepatic' transaminases, LDG (dose-dependent)
- peripheral hypostases, a hair loss (as a rule, it is restored after drug withdrawal)
- a vasculitis
- deterioration in hearing, paresthesia
- a polycystosis of ovaries
- disturbance of a menstrual cycle
- enuresis at children
Seldom
- constipations
- changes of behavior, mood or a mental state (depression, feeling of fatigue, a hallucination, aggression, a hyperactive state, psychoses, unusual excitement, motive concern or irritability), an ataxy, dizziness, drowsiness, a headache, encephalopathy, a dysarthtia, a stupor, disturbance of consciousness, a lump
- a leukopenia, a pancytopenia, an agranulocytosis
- abnormal liver functions
- a system lupus erythematosus
- a lethargy, confusion of consciousness
- increase in level of testosterone
- a headache, a nystagmus
- a reversible syndrome of parkinsonism
- skin rash, a small tortoiseshell, a Quincke's disease, a photosensitization
Very seldom
- encephalopathy, a coma
- pancreatitis, up to severe defeats with a lethal outcome (in the first 6 months of treatment, is more often on 2-12 weeks)
- a toxic epidermal necrolysis, Stephens-Johnson's syndrome, a polymorphic erythema
- a reversible syndrome of Fankoni
- a marrow aplasia
- a hyponatremia
-
the Contraindication renal failure
- hypersensitivity to Valproatum or any of fillers
- the profound abnormal liver functions and/or a pancreas
- a hepatic porphyria
- a case of heavy hepatitis in the personal or family anamnesis of the patient, including, connected with intake of medicines
- thrombocytopenia
- hemorrhagic diathesis
- the combined reception from karbapenema
- the combined reception with a St. John's wort
- the combined reception with meflokhiny
- children's age up to 3 months
- pregnancy and the period of a lactation
- to children up to 18 years at a maniacal-depressive syndrome with a bipolar course
- acute and chronic hepatitises
Medicinal interactions
At simultaneous use of valproic acid with the drugs oppressing central nervous system (tricyclic antidepressants, inhibitors of a monoaminooxidase (MAO) and neuroleptics) is possible strengthening of a depression of central nervous system. Ethanol, etc. gepatotoksichny means increase a likelihood of development of damages of a liver. The tricyclic antidepressants, MAO inhibitors, neuroleptics and other drugs reducing a threshold of convulsive activity reduce efficiency of valproic acid.
Convulexum, depending on its concentration in plasma, can vent thyroid hormones from places of their protein-binding of plasma and cause their metabolism which can lead to the false diagnosis indicating a hypothyroidism.
Other antiepileptic drugs about enzyme - the inducing action (Phenytoinum, phenobarbital, Primidonum, carbamazepine) reduce concentration of Valproatum in blood plasma. When performing combination therapy the dosage should be adjusted according to drug level in blood.
Simultaneous use with Convulexum of antidepressants, neuroleptics, tranquilizers, barbiturates, MAO inhibitors, timoleptik, ethanol is not recommended. Addition of Valproatum to clonazepam in isolated cases can lead to strengthening of expressiveness of the absansny status.
Valproatum can reduce metabolism of a lamotrigin and increase the average period of its semi-removal. Correcting of a dose (dose decline of a lamotrigin) can be required. Simultaneous use of a lamotrigin and Valproatum can increase risk of development (heavy) skin reactions, especially at children).
Valproatum can increase concentration of a zidovudine in blood plasma that will lead to increase in toxicity of the last.
At simultaneous use of valproic acid with barbiturates or Primidonum the increase in their concentration in blood plasma is noted. Increases elimination half-life (T1/2) of a lamotrigin (inhibits liver enzymes, causes delay of metabolism of a lamotrigin owing to what its T1/2 is extended up to 45-55 h - at children). Reduces clearance of a zidovudine by 38%, at this its T1/2 does not change.
At a combination to salicylates strengthening of effects of valproic acid is observed (replacement from communication with proteins of plasma). Convulexum enhances effect of antiagregant (acetylsalicylic acid) and indirect anticoagulants.
At a combination to phenobarbital, Phenytoinum, carbamazepine, meflokhiny valproic acid content in blood serum (metabolism acceleration) decreases.
Felbamat increases concentration of valproic acid in plasma by 35-50% (dose adjustment is necessary).
At the combined use of Cimetidinum or erythromycin. Concentration of Valproatum in blood plasma can increase (owing to reduction of his metabolism in a liver).
Holestiramin can lower valproic acid absorption.
At a concomitant use with Rifampitsinon the risk of attacks owing to the strengthened hepatic metabolism of Valproatum under the influence of rifampicin increases. Clinical and laboratory monitoring is recommended, dose adjustment of anticonvulsive drug is possible during treatment by rifampicin and after its cancellation.
Valproic acid does not cause induction of liver enzymes and does not reduce efficiency of oral contraceptives.
Special instructions
the Extra care is required when prescribing Convulexum to the following categories of patients:
- with anamnestic data on diseases of a liver and a pancreas and also on damage of marrow
- with renal failures
- with inborn enzymopathies
- to mentally retarded children
- at a hypoproteinemia
during drug treatment the alcohol intake is not allowed. Suicide thinking and behavior was noted among the patients receiving anti-epileptic substances according to some indications. The mechanism of emergence of such risk remains to unknown, and available data do not exclude the probability of increase in risk in view of intake of valproic acid.
In this regard patients have to be under careful observation regarding emergence of signs of suicide thinking and behavior, and the beginning of the corresponding treatment has to be considered. To patients (and to the persons who are looking after patients) it has to be reported that they have to see at once a doctor in case of suicide thinking and behavior.
At disturbances from a liver
Before an initiation of treatment and periodically within the first six months of treatment, especially among the patients entering into risk group and at what in the anamnesis liver diseases are noted constant control of parameters of function of a liver has to be exercised. Such patients have to be under careful medical observation.
Researches of function of a liver include definition of a prothrombin time, the levels of transaminase and/or bilirubin and/or products causing fibrinogen disintegration. At the first stage the increase in level of transaminase, this usually temporary phenomenon which answers a dose decline can be noted.
Patients with deviations have to pass in biochemical analyses repeated clinical assessment, and functions of a liver (including definition of a prothrombin time) have to be controlled until they are not normalized. Nevertheless, excessively extended prothrombin time, in particular, if it is connected with abnormal indicators of other relevant researches, demands the treatment termination.
Hepatic dysfunction, including the liver failure conducting by lethal outcomes is noted at patients whose treatment includes valproic acid or Valproatum of sodium. Patients who most often enter into risk group are children, in particular those which are younger than 3 years, and patients with the hereditary metabolic or degenerative disorders, organic brain dysfunction or serious epileptic seizures connected with a delay of intellectual development. The majority of these phenomena was noted during the first six months of therapy, mainly for 2 - 12 week and, as a rule, included anticonvulsant therapy from several drugs. For this group of patients the monotherapy is preferred.
At early stages of a liver failure the clinical symptoms can greatly help in updating of the diagnosis, than laboratory researches. Or with a lethal outcome uncharacteristic symptoms, usually with sudden emergence, such as loss of control over epileptic seizures, discomfort, weakness, lethargy, hypostasis, loss of appetite, vomiting, abdominal pain, drowsiness and jaundice can precede a hepatic disease of serious severity. They represent indications for immediate phase-out of medicine. Patients have to be instructed about that they at once reported about any such signs to the attending physician for carrying out the corresponding inspection. In spite of the fact that it is difficult to establish what inspections can give exact forecasts, it is considered that the inspections displaying protein synthesis, for example, a prothrombin time still are the most relevant.
At patients with hepatic dysfunction it is necessary to stop simultaneous use of salt of salicylic acid as she can use an identical metabolic way and, thereby, increase risk of appearance of a liver failure.
At hematologic disturbances
Before surgical intervention the general blood test (including numbers of thrombocytes), definition of a bleeding time, koagulogramma indicators is necessary. Patients in the history of whom damage of marrow is noted also have to be under careful control.
At disturbances from a pancreas
it was Seldom or never reported about pancreatitis of serious severity which could lead to a lethal outcome. The risk of a lethal outcome is noted most often at children of younger age and goes down with increase in age. Epileptic seizures of serious severity or disorder of neurologic character at use of the combined anticonvulsant therapy can represent risk factors of appearance of serious pancreatitis. Esli a renal failure will appear together with pancreatitis, the risk of a lethal outcome increases. Patients have to be informed that they have to see at once a doctor if they have symptoms indicating pancreatitis (for example, an abdominal pain, nausea, vomiting). Concerning such patients the careful medical assessment has to be carried out (including measurement of level of amylase in serum), when diagnosing pancreatitis the intake of Valproatum of sodium has to be stopped. Patients in whose anamnesis the pancreatitis is noted have to be under careful clinical observation.
In diabetes
during treatment it is necessary to consider possible distortion of results of analyses of urine in diabetes (owing to increase in maintenance of ketoproducts), indicators of function of a thyroid gland.
Increase in weight
Valproatum very often causes increase in weight which can be noticeable and progress. In an initiation of treatment to patients it has to be reported about such risk and also about the relevant activities for minimization of increase in weight.
Giperammoniyemiya
Esli is suspicions on enzymatic insufficiency of a cycle of ureapoiesis, metabolic researches before an initiation of treatment as there is a risk of emergence of a giperammoniyemiya at Valproatum use have to be conducted.
At development of any acute serious side effects it is necessary to discuss immediately with the doctor expediency of continuation or termination of treatment.
For reduction of risk of development of dispepsichesky disorders the intake of spasmolysants and the enveloping means is possible.
The sharp termination of intake of Convulexum can lead to increase of epileptic seizures.
Pregnancy
is not recommended intake of Convulexum during pregnancy and also the women of childbearing age who are not applying effective contraception.
The risk of the malformations caused by Valproatum is 3-4 times higher at the pregnant women taking this medicine, than the risk found in the general population which makes 3%. Most often observed malformations represent defects of closing of a neurotubule (approximately, 2-3%), dismorfiya of the person, a facial cleft, a craniostenosis, heart diseases, malformations of kidneys and urinary tract and deformation of extremities.
The doses exceeding 1000 mg/day and combination with other anticonvulsant drugs are important risk factors of forming of malformations at a fruit.
Modern epidemiological data do not indicate decrease in overal coefficient of intellectual development of children on exposure by sodium Valproatum.
However, at such children some decrease in verbal abilities and/or more frequent address to logopedists or behind additional classes is described. Besides, several cases of autism and the related disturbances are registered at the children who transferred exposure by sodium Valproatum in a pre-natal state. Additional researches are necessary for confirmation or a denial of these results.
When planning pregnancy
If pregnancy is planned, it is necessary to resolve by all means an issue of use of other medical drugs.
If use of Valproatum of sodium is inevitable (i.e. there is no other alternative), it is recommended to appoint the minimum effective daily dose. It is necessary to apply dosage forms of the prolonged release or - if it it is impossible - to distribute a daily dose on several receptions. It is necessary to avoid the peaks of the maximum concentration of valproic acid in blood plasma.
Considering favorable effect of folic acid before pregnancy emergence, it is possible to offer additional intake of folic acid in a dose of 5 mg/day in 1 month prior to conception and within 2 months after it. The inspection directed to detection of malformations has to be identical to all irrespective of whether the pregnant woman accepts folic acid or not.
During pregnancy:
If the choice of other drug is absolutely impossible, and it is necessary to continue treatment by sodium Valproatum, it is recommended to appoint the minimum effective dose. It is necessary to avoid whenever possible purpose of the doses exceeding 1000 mg/day. Irrespective of intake of folic acid, inspection on presence of malformations at a fruit is necessary for all pregnant women.
Before childbirth the koagulogramm should make, in particular, number of thrombocytes, level of fibrinogen and a blood clotting time (the activated partial tromboplastinovy time, APTV).
Newborns
Convulexum can cause development of the hemorrhagic syndrome in newborns which is not connected with deficiency of vitamin K.
Normal indicators of a hemostasis of mother do not exclude a possibility of pathology at the newborn. Therefore, at the newborn it is necessary to define number of thrombocytes, level of fibrinogen and the activated partial tromboplastinovy time (APTT). At newborns hypoglycemia cases in the first week of life are also registered.
The lactation
Valproatum is allocated with breast milk in a small amount (1-10% of drug level in blood plasma of mother). However, in connection with data on reduced verbal abilities at children of younger age it is necessary to advise patients to refuse breastfeeding.
The feature of influence on ability to run the vehicle or potentially dangerous mechanisms
is not recommended to be engaged in the types of activity requiring special attention and high speed of psychomotor reactions (driving of motor transport and control of mechanisms).
Overdose
Symptoms: nausea, vomiting, dizziness, diarrhea, breath dysfunction, hypomyotonia, hyporeflexia, miosis, coma.
Treatment: gastric lavage (not later than 10-12 h), activated carbon, Naloxonum in/in, the hemodialysis, hemoperfusion, an artificial diuresis, maintenance of breath and functions of the cardiovascular
Release Form system and packing
On 100 ml of drug place in bottles of amber glass with the red or white fixed screwing-up cover from polyethylene of high pressure and control of the first opening.
On a bottle paste the label self-adhesive.
On 1 bottle together with the portioning device and the instruction for medical use in the state and Russian languages put in a pack from cardboard.
To Store storage conditions at a temperature not over 250C, in the dry, protected from light place.
To store out of children's reach!
A period of storage
of 5 years
the use Period after opening of a bottle – no more than 6 months.
Not to use drug after expiry date.
Prescription status
According to the prescription
the Producer
G.L. Pharma GmbH., Austria, A-1160, Vienna, Arnetgasse 3
the Owner of the registration certificate
of OOO Valeant, Russia
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products Representative office of OOO Valeant to RKKazahstan, 050059, Almaty, Al-Farabi Avenue, 17, Business center Nurly-Tau Block 4B, office 1104 Phone number + 7 727 3 111 516, fax +7 727 3 111 517 E-mail:
To Develop Îffice.KZ@valeant.com
of Konvuleks® medicine
the Trade name
of Konvuleks®
the International unlicensed
name Valproic Acid Dosage Form
of the Drop oral
Structure
of 1 ml of solution contains
active agent - sodium Valproatum of 300 mg, (it is equivalent to valproic acid of 260.30 mg and sodium to hydroxide of 72.20 mg),
excipients: sodium saccharin, fragrance orange, sodium hydroxide, acid chlorohydrogen, the water purified
the Description
Transparent solution from colourless till slightly yellowish color with a sweet orange flavor and easy burning aftertaste.
Pharmacotherapeutic group
Antiepileptic drugs. Fatty acids derivatives. Valproic acid.
The ATX N03AG01 code
the Pharmacological
Pharmacokinetics Valproic Acid properties quickly and almost is completely soaked up in a GIT, the bioavailability at intake is 100%. Meal does not reduce absorption speed. The maximum level of concentration in plasma is noted in 3-4 h. Equilibrium concentration is reached for 2-4 day of treatment, depending on dosing intervals. Therapeutic concentration of drug in blood plasma fluctuates within 50-150 mg/l. Valproic acid is connected with proteins of plasma for 90-95% at concentration in blood plasma up to 50 mg/l and for 80-85% at concentration of 50-100 mg/l, in uraemia, a hypoproteinemia and cirrhosis the linking with proteins is reduced. Concentration levels in cerebrospinal fluid correlate with the size of the fraction of drug which is not connected with proteins. Valproic acid gets through a placental barrier, is emitted with breast milk. Concentration in breast milk makes 1-10% of concentration in blood plasma of mother. Drug is exposed to a glyukuronirovaniye and oxidation in a liver, metabolites and not changed valproic acid (1-3% of a dose) are removed by kidneys, small amounts are removed with excrements and with expired air. Elimination half-life of drug makes at healthy examinees and at monotherapy from 8 to 24 h, at a combination to other medicines the elimination half-life can make 6-8 h owing to induction of metabolic enzymes, at patients with an abnormal liver function and elderly patients can be much longer.
The prolonged form is characterized by lack of latent time of absorption, slow absorption, lower (for 25%), but rather more stable concentration in plasma between 4 and 14 h.
The pharmacodynamics
Convulexum – antiepileptic means, also has the central myorelaxation and sedative effect. The mechanism of action is caused mainly by inhibition of enzyme of GAMK-transferase and increase in content of piperidic acid (GAMK) in the central nervous system (CNS). GAMK interferes pre- and to postsynaptic categories and, thereby, prevents distribution of convulsive activity to central nervous system. Besides, in the mechanism of effect of drug the essential role belongs to impact of valproic acid on GAMK A receptors and also influence on a voltage - dependent Na-channels. Affects sites of postsynaptic receptors, imitating or enhancing the braking effect of GAMK. Potential direct impact on activity of membranes is connected with changes in conductivity of potassium. Improves a mental condition and mood of patients, has antiarrhytmic activity.
Indications
- epileptic seizures (including generalized and partial and also against the background of organic diseases of a brain)
- prevention of attacks of migraine
- the manic-depressive syndrome with a bipolar course when lithium is contraindicated or is not transferred by the patient
the Route of administration and doses
take the Drug inside, 2-3 times a day, in time or after a meal, with a small amount of liquid.
Adults. An initial dose at monotherapy - 5-15 mg/kg/days, at combination therapy – 10-30 mg/kg/days then gradually increase this dose by 5-10 mg/kg/week.
An average daily dose - 20-30 mg/kg of body weight.
The daily dose can be increased up to 60 mg/kg at a possibility of the organization of control of concentration of drug in blood plasma.
Children to the 6th summer age. An average daily dose at monotherapy – 15-45 mg/kg, maximum – 50mg/kg. At combination therapy of-30-100 mg/kg/days.
The dosage at children of 6 years is also more senior makes 5-15 mg/kg with gradual increase up to 20-30 mg/kg a day. At children who need a dose higher than 40 mg/kg a day biochemical and hematologic parameters have to be traced.
Average daily doses:
Age
Body weight
the Average dose
(in kg)
(mg/day)
of ml/day
of 3 - 6 months
5.5 -
7.5 150 0.5 ml
of 6 - 12 months
7.5 - 10,150 -
300 0.5 - 1 ml
of 1 - 3 years
10 - 15
300 - 450 1 - 1.5 ml
of 3 - 6 years
15 -
20 450 - 600 1.5 - 2 ml
of 7 - 11 years
of
20 - 40 600 - 1200 2 - 4 ml
of 12 - 17 years
40 - 60 1000 - 1500
ca. 3.5 - 5 ml
Adult (including elderly)
³
60 1200 - 2100 4 - 7 ml
Advanced age. Though the pharmacokinetics of Valproatums at advanced age has the features, it has limited clinical value, and the dose should be determined by clinical effect. Owing to reduction of linking with seralbumin the share of untied drug in plasma increases. It causes expediency of more careful selection of a dose of drug at elderly, with possible use of smaller doses of drug.
Patients with a renal failure. It can be necessary to lower a drug dose. The dose should be selected for monitoring of a clinical state as concentration indicators in plasma can be insufficiently informative.
Use of the portioning device.
1. To lower the piston in the syringe down, against the stop, then to place the syringe in a glass small bottle.
2. To lift the piston up until the mark in the piston corresponds to the ordered dosage (graduation in ml and mg). If necessary to repeat process before achievement of the general appointed quantity.
3. By means of pressing of the piston down, apply the measured dose in a small amount of liquid.
4. After each use, close a small bottle, and carefully rinse the syringe with water. You store both the syringe and a small bottle in a cardboard box.
Side effects
Side effects are possible generally at drug level in plasma higher than 100 mg/l or at the combined therapy.
Often
- nausea, vomiting, anorexia or increase in appetite, diarrhea, gastralgias, hepatitis
- a tremor
- a diplopia, flashing of 'front sights' before eyes
- anemia, thrombocytopenia, the decrease in content of fibrinogen, aggregation of thrombocytes and blood clotting which is followed by lengthening of a bleeding time, petekhialny hemorrhages, bruises, hematomas, bleeding, an agranulocytosis, a lymphocytosis
- decrease or increase in body weight
- a giperkreatininemiya, a giperammoniyemiya, a hyperbilirubinemia, insignificant increase in activity of 'hepatic' transaminases, LDG (dose-dependent)
- peripheral hypostases, a hair loss (as a rule, it is restored after drug withdrawal)
- a vasculitis
- deterioration in hearing, paresthesia
- a polycystosis of ovaries
- disturbance of a menstrual cycle
- enuresis at children
Seldom
- constipations
- changes of behavior, mood or a mental state (depression, feeling of fatigue, a hallucination, aggression, a hyperactive state, psychoses, unusual excitement, motive concern or irritability), an ataxy, dizziness, drowsiness, a headache, encephalopathy, a dysarthtia, a stupor, disturbance of consciousness, a lump
- a leukopenia, a pancytopenia, an agranulocytosis
- abnormal liver functions
- a system lupus erythematosus
- a lethargy, confusion of consciousness
- increase in level of testosterone
- a headache, a nystagmus
- a reversible syndrome of parkinsonism
- skin rash, a small tortoiseshell, a Quincke's disease, a photosensitization
Very seldom
- encephalopathy, a coma
- pancreatitis, up to severe defeats with a lethal outcome (in the first 6 months of treatment, is more often on 2-12 weeks)
- a toxic epidermal necrolysis, Stephens-Johnson's syndrome, a polymorphic erythema
- a reversible syndrome of Fankoni
- a marrow aplasia
- a hyponatremia
-
the Contraindication renal failure
- hypersensitivity to Valproatum or any of fillers
- the profound abnormal liver functions and/or a pancreas
- a hepatic porphyria
- a case of heavy hepatitis in the personal or family anamnesis of the patient, including, connected with intake of medicines
- thrombocytopenia
- hemorrhagic diathesis
- the combined reception from karbapenema
- the combined reception with a St. John's wort
- the combined reception with meflokhiny
- children's age up to 3 months
- pregnancy and the period of a lactation
- to children up to 18 years at a maniacal-depressive syndrome with a bipolar course
- acute and chronic hepatitises
Medicinal interactions
At simultaneous use of valproic acid with the drugs oppressing central nervous system (tricyclic antidepressants, inhibitors of a monoaminooxidase (MAO) and neuroleptics) is possible strengthening of a depression of central nervous system. Ethanol, etc. gepatotoksichny means increase a likelihood of development of damages of a liver. The tricyclic antidepressants, MAO inhibitors, neuroleptics and other drugs reducing a threshold of convulsive activity reduce efficiency of valproic acid.
Convulexum, depending on its concentration in plasma, can vent thyroid hormones from places of their protein-binding of plasma and cause their metabolism which can lead to the false diagnosis indicating a hypothyroidism.
Other antiepileptic drugs about enzyme - the inducing action (Phenytoinum, phenobarbital, Primidonum, carbamazepine) reduce concentration of Valproatum in blood plasma. When performing combination therapy the dosage should be adjusted according to drug level in blood.
Simultaneous use with Convulexum of antidepressants, neuroleptics, tranquilizers, barbiturates, MAO inhibitors, timoleptik, ethanol is not recommended. Addition of Valproatum to clonazepam in isolated cases can lead to strengthening of expressiveness of the absansny status.
Valproatum can reduce metabolism of a lamotrigin and increase the average period of its semi-removal. Correcting of a dose (dose decline of a lamotrigin) can be required. Simultaneous use of a lamotrigin and Valproatum can increase risk of development (heavy) skin reactions, especially at children).
Valproatum can increase concentration of a zidovudine in blood plasma that will lead to increase in toxicity of the last.
At simultaneous use of valproic acid with barbiturates or Primidonum the increase in their concentration in blood plasma is noted. Increases elimination half-life (T1/2) of a lamotrigin (inhibits liver enzymes, causes delay of metabolism of a lamotrigin owing to what its T1/2 is extended up to 45-55 h - at children). Reduces clearance of a zidovudine by 38%, at this its T1/2 does not change.
At a combination to salicylates strengthening of effects of valproic acid is observed (replacement from communication with proteins of plasma). Convulexum enhances effect of antiagregant (acetylsalicylic acid) and indirect anticoagulants.
At a combination to phenobarbital, Phenytoinum, carbamazepine, meflokhiny valproic acid content in blood serum (metabolism acceleration) decreases.
Felbamat increases concentration of valproic acid in plasma by 35-50% (dose adjustment is necessary).
At the combined use of Cimetidinum or erythromycin. Concentration of Valproatum in blood plasma can increase (owing to reduction of his metabolism in a liver).
Holestiramin can lower valproic acid absorption.
At a concomitant use with Rifampitsinon the risk of attacks owing to the strengthened hepatic metabolism of Valproatum under the influence of rifampicin increases. Clinical and laboratory monitoring is recommended, dose adjustment of anticonvulsive drug is possible during treatment by rifampicin and after its cancellation.
Valproic acid does not cause induction of liver enzymes and does not reduce efficiency of oral contraceptives.
Special instructions
the Extra care is required when prescribing Convulexum to the following categories of patients:
- with anamnestic data on diseases of a liver and a pancreas and also on damage of marrow
- with renal failures
- with inborn enzymopathies
- to mentally retarded children
- at a hypoproteinemia
during drug treatment the alcohol intake is not allowed. Suicide thinking and behavior was noted among the patients receiving anti-epileptic substances according to some indications. The mechanism of emergence of such risk remains to unknown, and available data do not exclude the probability of increase in risk in view of intake of valproic acid.
In this regard patients have to be under careful observation regarding emergence of signs of suicide thinking and behavior, and the beginning of the corresponding treatment has to be considered. To patients (and to the persons who are looking after patients) it has to be reported that they have to see at once a doctor in case of suicide thinking and behavior.
At disturbances from a liver
Before an initiation of treatment and periodically within the first six months of treatment, especially among the patients entering into risk group and at what in the anamnesis liver diseases are noted constant control of parameters of function of a liver has to be exercised. Such patients have to be under careful medical observation.
Researches of function of a liver include definition of a prothrombin time, the levels of transaminase and/or bilirubin and/or products causing fibrinogen disintegration. At the first stage the increase in level of transaminase, this usually temporary phenomenon which answers a dose decline can be noted.
Patients with deviations have to pass in biochemical analyses repeated clinical assessment, and functions of a liver (including definition of a prothrombin time) have to be controlled until they are not normalized. Nevertheless, excessively extended prothrombin time, in particular, if it is connected with abnormal indicators of other relevant researches, demands the treatment termination.
Hepatic dysfunction, including the liver failure conducting by lethal outcomes is noted at patients whose treatment includes valproic acid or Valproatum of sodium. Patients who most often enter into risk group are children, in particular those which are younger than 3 years, and patients with the hereditary metabolic or degenerative disorders, organic brain dysfunction or serious epileptic seizures connected with a delay of intellectual development. The majority of these phenomena was noted during the first six months of therapy, mainly for 2 - 12 week and, as a rule, included anticonvulsant therapy from several drugs. For this group of patients the monotherapy is preferred.
At early stages of a liver failure the clinical symptoms can greatly help in updating of the diagnosis, than laboratory researches. Or with a lethal outcome uncharacteristic symptoms, usually with sudden emergence, such as loss of control over epileptic seizures, discomfort, weakness, lethargy, hypostasis, loss of appetite, vomiting, abdominal pain, drowsiness and jaundice can precede a hepatic disease of serious severity. They represent indications for immediate phase-out of medicine. Patients have to be instructed about that they at once reported about any such signs to the attending physician for carrying out the corresponding inspection. In spite of the fact that it is difficult to establish what inspections can give exact forecasts, it is considered that the inspections displaying protein synthesis, for example, a prothrombin time still are the most relevant.
At patients with hepatic dysfunction it is necessary to stop simultaneous use of salt of salicylic acid as she can use an identical metabolic way and, thereby, increase risk of appearance of a liver failure.
At hematologic disturbances
Before surgical intervention the general blood test (including numbers of thrombocytes), definition of a bleeding time, koagulogramma indicators is necessary. Patients in the history of whom damage of marrow is noted also have to be under careful control.
At disturbances from a pancreas
it was Seldom or never reported about pancreatitis of serious severity which could lead to a lethal outcome. The risk of a lethal outcome is noted most often at children of younger age and goes down with increase in age. Epileptic seizures of serious severity or disorder of neurologic character at use of the combined anticonvulsant therapy can represent risk factors of appearance of serious pancreatitis. Esli a renal failure will appear together with pancreatitis, the risk of a lethal outcome increases. Patients have to be informed that they have to see at once a doctor if they have symptoms indicating pancreatitis (for example, an abdominal pain, nausea, vomiting). Concerning such patients the careful medical assessment has to be carried out (including measurement of level of amylase in serum), when diagnosing pancreatitis the intake of Valproatum of sodium has to be stopped. Patients in whose anamnesis the pancreatitis is noted have to be under careful clinical observation.
In diabetes
during treatment it is necessary to consider possible distortion of results of analyses of urine in diabetes (owing to increase in maintenance of ketoproducts), indicators of function of a thyroid gland.
Increase in weight
Valproatum very often causes increase in weight which can be noticeable and progress. In an initiation of treatment to patients it has to be reported about such risk and also about the relevant activities for minimization of increase in weight.
Giperammoniyemiya
Esli is suspicions on enzymatic insufficiency of a cycle of ureapoiesis, metabolic researches before an initiation of treatment as there is a risk of emergence of a giperammoniyemiya at Valproatum use have to be conducted.
At development of any acute serious side effects it is necessary to discuss immediately with the doctor expediency of continuation or termination of treatment.
For reduction of risk of development of dispepsichesky disorders the intake of spasmolysants and the enveloping means is possible.
The sharp termination of intake of Convulexum can lead to increase of epileptic seizures.
Pregnancy
is not recommended intake of Convulexum during pregnancy and also the women of childbearing age who are not applying effective contraception.
The risk of the malformations caused by Valproatum is 3-4 times higher at the pregnant women taking this medicine, than the risk found in the general population which makes 3%. Most often observed malformations represent defects of closing of a neurotubule (approximately, 2-3%), dismorfiya of the person, a facial cleft, a craniostenosis, heart diseases, malformations of kidneys and urinary tract and deformation of extremities.
The doses exceeding 1000 mg/day and combination with other anticonvulsant drugs are important risk factors of forming of malformations at a fruit.
Modern epidemiological data do not indicate decrease in overal coefficient of intellectual development of children on exposure by sodium Valproatum.
However, at such children some decrease in verbal abilities and/or more frequent address to logopedists or behind additional classes is described. Besides, several cases of autism and the related disturbances are registered at the children who transferred exposure by sodium Valproatum in a pre-natal state. Additional researches are necessary for confirmation or a denial of these results.
When planning pregnancy
If pregnancy is planned, it is necessary to resolve by all means an issue of use of other medical drugs.
If use of Valproatum of sodium is inevitable (i.e. there is no other alternative), it is recommended to appoint the minimum effective daily dose. It is necessary to apply dosage forms of the prolonged release or - if it it is impossible - to distribute a daily dose on several receptions. It is necessary to avoid the peaks of the maximum concentration of valproic acid in blood plasma.
Considering favorable effect of folic acid before pregnancy emergence, it is possible to offer additional intake of folic acid in a dose of 5 mg/day in 1 month prior to conception and within 2 months after it. The inspection directed to detection of malformations has to be identical to all irrespective of whether the pregnant woman accepts folic acid or not.
During pregnancy:
If the choice of other drug is absolutely impossible, and it is necessary to continue treatment by sodium Valproatum, it is recommended to appoint the minimum effective dose. It is necessary to avoid whenever possible purpose of the doses exceeding 1000 mg/day. Irrespective of intake of folic acid, inspection on presence of malformations at a fruit is necessary for all pregnant women.
Before childbirth the koagulogramm should make, in particular, number of thrombocytes, level of fibrinogen and a blood clotting time (the activated partial tromboplastinovy time, APTV).
Newborns
Convulexum can cause development of the hemorrhagic syndrome in newborns which is not connected with deficiency of vitamin K.
Normal indicators of a hemostasis of mother do not exclude a possibility of pathology at the newborn. Therefore, at the newborn it is necessary to define number of thrombocytes, level of fibrinogen and the activated partial tromboplastinovy time (APTT). At newborns hypoglycemia cases in the first week of life are also registered.
The lactation
Valproatum is allocated with breast milk in a small amount (1-10% of drug level in blood plasma of mother). However, in connection with data on reduced verbal abilities at children of younger age it is necessary to advise patients to refuse breastfeeding.
The feature of influence on ability to run the vehicle or potentially dangerous mechanisms
is not recommended to be engaged in the types of activity requiring special attention and high speed of psychomotor reactions (driving of motor transport and control of mechanisms).
Overdose
Symptoms: nausea, vomiting, dizziness, diarrhea, breath dysfunction, hypomyotonia, hyporeflexia, miosis, coma.
Treatment: gastric lavage (not later than 10-12 h), activated carbon, Naloxonum in/in, the hemodialysis, hemoperfusion, an artificial diuresis, maintenance of breath and functions of the cardiovascular
Release Form system and packing
On 100 ml of drug place in bottles of amber glass with the red or white fixed screwing-up cover from polyethylene of high pressure and control of the first opening.
On a bottle paste the label self-adhesive.
On 1 bottle together with the portioning device and the instruction for medical use in the state and Russian languages put in a pack from cardboard.
To Store storage conditions at a temperature not over 250C, in the dry, protected from light place.
To store out of children's reach!
A period of storage
of 5 years
the use Period after opening of a bottle – no more than 6 months.
Not to use drug after expiry date.
Prescription status
According to the prescription
the Producer
G.L. Pharma GmbH., Austria, A-1160, Vienna, Arnetgasse 3
the Owner of the registration certificate
of OOO Valeant, Russia
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products Representative office of OOO Valeant to RKKazahstan, 050059, Almaty, Al-Farabi Avenue, 17, Business center Nurly-Tau Block 4B, office 1104 Phone number + 7 727 3 111 516, fax +7 727 3 111 517 E-mail:
To Develop Îffice.KZ@valeant.com