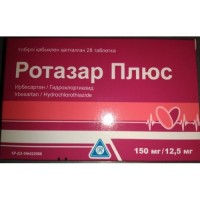
Ko Irbesan 150 mg / 12.5 mg 14s film-coated tablets
- $20.40
Sku:
60bfbe56a97b
Brand:
Nobel AFF (Kazakhstan)
The instruction for medical use
of KO-IRBESAN medicine
A trade name
of KO-IRBESAN
the International unlicensed name
Is not present
the Dosage form of the Tablet, film coated, 150 mg / 12.5 mg and 300 mg / 12.5 mg
Structure
One tablet contains
active agent irbesartan 150.00 mg or 300.00 mg
Hydrochlorthiazidum of 12.50 mg,
excipients: tselaktoza 80, cellulose microcrystalline PH 102, starch prezhelatinizirovanny 1500, sodium of a kroskarmelloz, aerosil 200, magnesium stearate, ferrous oxide red, ferrous oxide yellow,
structure of a cover: the gipromeloza, the titan dioxide, carmoisine Aluminium Lake (E 122), FD & C yellow #6 sanst yellow (E110), FD & C blue #2 Al Indigo carmine (E132), ferrous oxide yellow (E172), hydroxypropyl cellulose, water cleaned
the Description
of the Tablet, film coated pink color, an oval form, biconvex and from risky on one party (for a dosage of 150 mg / 12.5 mg).
Tablets, film coated pink color, oblong shape, biconvex and with profound risky on one party (for a dosage of 300 mg / 12.5 mg).
Pharmacotherapeutic group
the Drugs influencing a system renin-angiotensin.
Angiotensin II antagonists in a combination with diuretics.
Code of automatic telephone exchange C09DA04
Pharmacological
Pharmacokinetics Combined properties antihypertensive drug. After intake irbesartan and Hydrochlorthiazidum are well soaked up. The absolute bioavailability of an irbesartan is about 60 - 80%. The maximum concentration of an irbesartan in blood plasma is reached in 1.5 - 2 h, reaching up to 80 - 85%. The concomitant use of food significantly does not affect bioavailability of an irbesartan. Linking of an irbesartan with proteins of blood plasma makes about 96% at insignificant linking with cellular components of blood. Elimination half-life of an irbesartan in a terminal phase makes 11 - 15 h. Equilibrium concentration of an irbesartan in blood plasma is reached within 3 days since the beginning of use of drug at reception of 1 times a day. The linear dependence on a dose in the range of doses from 10 to 600 mg is characteristic of pharmacokinetic indicators of an irbesartan. Weaker, than proportional, increase at absorption was observed at intake in doses more than 600 mg. At repeated receptions of 1 times a day the limited accumulation of an irbesartan in blood plasma is noted (& lt, 20%). The general clearance of an irbesartan of an organism and renal clearance is made by 157 - 176 and 3 - 3.5 ml/min. respectively. Irbesartan is metabolized in a liver by conjugation with formation of a glucuronide and oxidation. The main metabolite circulating in blood is the irbesartana a glucuronide (about 6%). Irbesartan is oxidized mainly with participation of CYP P450 cytochrome 2C9 while the isoenzyme of CYP 3A4 is of little importance. Irbesartan and his metabolites are brought out of an organism both with bile, and with urine. About 20% are removed with urine, and the rest - with a stake. Less than 2% of the accepted dose are allocated with urine in the form of not changed irbesartan.
Hydrochlorthiazidum at oral administration is pretty fast soaked up from digestive tract. The bioavailability is about 60 - 80%. During 24 h in not changed look, at least, 61% of an oral dose are removed. Hydrochlorthiazidum is not metabolized, and quickly emitted with kidneys. Gets through a placental barrier, but does not get through a blood-brain barrier (GEB). The diuretic effect develops in 2 hours after oral administration, reaches a maximum approximately by the 4th o'clock and lasts from 5.6 to 14.8 hours. Elimination half-life makes of 6 - 10 o'clock.
The pharmacodynamics
of KO-IRBESAN is a combination irbesartana-the selection antagonist of receptors of angiotensin ІІ (AT1 type) and Hydrochlorthiazidum - diuretic of a class of tiazid. At combined use of an irbesartan and Hydrochlorthiazidum the antihypertensive effect increases. Irbesartan blocks all physiologically significant effects of angiotensin II which are implemented through AT1 receptors irrespective of a source or a way of synthesis of angiotensin II. Specific antagonistic action of an irbesartan concerning receptors of angiotensin II (AT1) leads to increase in concentration of renin and angiotensin II in blood plasma and to decrease in concentration of Aldosteronum in blood plasma. When using drug in the recommended dose the concentration of potassium ions in blood serum significantly does not change. Does not inhibit APF (a kininaz of II - the enzyme which is responsible for formation of angiotensin II), causes metabolic degradation of bradykinin with formation of inactive metabolites. Manifestation of effect does not require metabolic activation. CO-Irbesan lowers the arterial blood pressure at the minimum change of the heart rate (HR). Decrease in the ABP at reception of 1 times a day has dose-dependent character, with a tendency to an exit to the plateau when assigning in doses more than 300 mg. Purpose of 1 tablet of 1 times a day causes decrease in the ABP during 24 h after administration of drug (in a prone position or sitting). The maximum decrease in the ABP level is reached in 3 - 6 h after administration of drug, the hypotensive effect remains at least during 24 h. In 24 h after reception in the recommended doses the decrease in the ABP is 50 - 70% in comparison with the maximum level of decrease in diastolic and systolic arterial blood pressure. Administration of drug of KO-IRBESAN in a dosage of 150 mg/12.5 mg of 1 times a day is accorded by the effect similar to that which is reached at division of this daily dose into 2 receptions. At regular use during 12 weeks the effect gains stability and in 46 weeks reaches a maximum. The hypotensive effect remains at long-term treatment. After the termination of treatment of the ABP gradually returns to initial level.
Hydrochlorthiazidum affects mechanisms of a reabsorption of electrolytes in renal tubules, directly strengthening discharge of ions of sodium and chlorine in approximately identical quantities. Indirectly, as a result of diuretic activity of Hydrochlorthiazidum, blood plasma volume decreases therefore the activity of renin increases in plasma, secretion of Aldosteronum increases, loss of potassium increases in urine and potassium content in blood serum decreases. The hydrochlorothiazide is thiazide diuretic of tiazid and is used at treatment of hypertensia and hypostases. Thiazide diuretics influence renal mechanisms of a reabsorption of electrolytes, increasing excretion of sodium and chlorine
Dov in approximately equivalent quantities, brake a sodium reabsorption. The mechanism of action is connected with influence on distal tubules of nephron where the reabsorption of ions of sodium, chlorine and water decreases. Excretion of potassium ions, magnesium and bicarbonate increase, calcium ions are late in an organism. The hydrochlorothiazide reduces plasma volume, increasing activity of renin in plasma and secretion of Aldosteronum, with the subsequent increase in content of potassium in urine and decrease in its content in blood serum. By means of blockade the combined use of an irbesartan leads system renin-angiotensin-aldosteronovoy to prevention of loss of potassium in the blood serum caused by this diuretic.
At the same time diuretics of group of tiazid render hypotensive effect by means of decrease in peripheric resistance.
At combined use Hydrochlorthiazidum not only strengthens, but also angiotensin II extends antihypertensive action of blockers of receptors. Besides, combined use of blockers of receptors and thiazide diuretic drugs leads angiotensin II to noticeable decrease in the ABP at patients both with high, and with low activity of renin, and the response to this combination therapy makes about 80% and above.
Indications - essetsialny arterial hypertension - arterial hypertension symptomatic, including at patients with diseases of kidneys and diabetes ІІ type (as a part of antihypertensive therapy)
the Route of administration and doses
Adult Patients from whom it is not possible to achieve adequate control of arterial blood pressure at monotherapy irbesartany or Hydrochlorthiazidum, can be transferred to administration of drug of KO IRBESAN.
To patients with the arterial blood pressure (ABP) more than 160/100 mm Hg at a combination to diabetes or/and chronic kidney disease the combination therapy can be appointed on start of treatment from purpose of 1 tablet KO-IRBESAN in a dosage of 150 mg/12.5 mg of 1 times a day which, if necessary, is raised subsequently to 300 mg / 12.5мг 1 time in day.
An initial and maintenance dose - 1 tablet TO - IRBESAN in a dosage of 150 mg / 12.5 mg a day regardless of meal. The effective dose of Hydrochlorthiazidum usually makes 12.5 mg/days.
KO-IRBESAN in a dosage of 150 mg / 12.5 usually provides mg of 1 times a day optimum 24 - hour control of the ABP. At insufficient efficiency of a dose of 150 mg / 12.5 mg of drug KO-IRBESAN of 1 times a day appoint 300 mg / 12.5мг drug.
Use of drug in doses of more than 300 mg irbesartana/25 is not recommended to mg of a hydrochlorothiazide of 1 times/days.
Side effects
- dizziness, orthostatic dizziness, the general weakness
- orthostatic hypotension
- nausea, vomiting, the dispeptic phenomena, heartburn
- cough, rhinitis, pharyngitis
- musculoskeletal pains (myalgias, ossalgiya, thorax pains)
- increase in the KFK level in blood plasma, a hyperpotassemia
Seldom
- a headache, a ring in ears, fatigue, a condition of alarm / excite
pave
- tachycardia, rushes of blood
- rash, urticaria, a Quincke's disease
- disturbance of flavoring feelings
- an abnormal liver function, hepatitis
- an abdominal cavity pains
- infections of urinary tract, a renal failure (in edinich
ny cases - a renal failure at patients of group of the increased
risk)
- decrease in level of hemoglobin
of the Contraindication
- hypersensitivity to drug components
- primary hyper aldosteronism
- a hypovolemia (including against the background of high doses of diuretics)
- an anury
- pregnancy and the period of a lactation
- children's and teenage age up to 18 years
Medicinal interaction At simultaneous use of KO-IRBESAN with: - thiazide diuretics, the antihypertensive effect of an irbesartan amplifies. the previous treatment by diuretics in high doses can lead to dehydration and increases risk of developing symptomatic hypotension, - with the kaliysberegayushchy diuretics and drugs of potassium, substitutes of table salt and other drugs increasing potassium level in blood serum (for example heparin), risk of development of a hyperpotassemia increases,
- other antihypertensive drugs, the hypotensive effect amplifies, we will combine with beta blockers, blockers of calcium channels, digoxin, Cimetidinum, - lithium drugs, increase in concentration of lithium in blood plasma is possible and development of its toxic effects therefore such combination is not recommended if such combination is necessary, is recommended to carry out careful control of level of lithium in blood serum, - warfarin, tolbutamide (CYP substrates 2C9) and also nifedipine (CYP inhibitor 2C9), noticeable pharmacokinetic or pharmakodinamichesky interactions are not revealed, KO-IRBESAN can be combined safely with blockers ‑ adrenoceptors, antagonists of calcium of long action and thiazide diuretics. TO - IRBESAN in a dosage of 150 mg / 12.5мг does not influence digoxin pharmacokinetics. Operation of inductors CYP 2C9, such as rifampicin, is unknown.
Special instructions At patients with dehydration and at patients with a hyponatremia who resulted from intensive treatment by diuretics of diarrhea, vomiting or limited consumption of salt, symptomatic hypotension, especially after reception of the first dose of drug can develop. The hypovolemia and a hyponatremia have to be eliminated prior to use of drug KO-IRBESAN. Patients with a bilateral stenosis of renal arteries or a stenosis of an artery of the only functioning kidney who take other drugs influencing renin - angiotensin - an aldosteronovy system treat group of the increased risk concerning development of heavy arterial hypotension or a renal failure. Though such cases for drug TO - IRBESAN are not described, the similar effect can be expected when using antagonists of receptors of angiotensin II. As well as in case of prescribing of any other hypotensive drug, excessive decrease in the ABP at patients with an ischemic cardiopathy or an ischemic heart disease can lead to a myocardial infarction or a stroke. As well as when using other drugs influencing renin-angio-tenzin-aldosteronovuyu a system during the KO-IRBESAN drug treatment the hyperpotassemia, especially in the presence of a renal failure, the proteinuria caused by a diabetic nephropathy and/or heart diseases can be observed. For identification of a hyperpotassemia during drug treatment the monitoring of level of potassium and creatinine in blood serum is recommended. As well as when using other vazodilatator, it is necessary to take special precautionary measures for patients with an aortal or mitral stenosis or obstructive hypertrophic kardiomio
a patiya. At patients, the vascular tone and which function of kidneys depend mainly on activity system renin-angiotensin-aldosteronovoy (for example at severe forms of stagnant heart failure or diseases of kidneys, including a renal artery stenosis), treatment by APF inhibitors or antagonists of receptors of angiotensin ІІ can cause acute arterial hypotension, an azotemia, an oliguria and in rare instances - OPN. There are no clinical data on use of drug KO-IRBESAN for the patients who transferred transplantation of kidneys. At establishment of pregnancy of KO-IRBESAN it is necessary to cancel as soon as possible. For detection of malformations it is recommended to carry out ultrasonography of a fruit. It is unknown whether it is excreted irbesartan in breast milk. Use in pediatrics Safety and efficiency of drug at children are not established. The feature of influence of medicine on ability to run vehicles and potentially dangerous mechanisms Should pay attention to a possibility of development of side effects (for example, dizzinesses) which can affect ability to run the vehicle or potentially dangerous mechanisms. Patients need to be warned about danger of performance of the work requiring special attention and speed of psychomotor reactions before disappearance of these side effects.
The overdose
to Develop
of KO-IRBESAN medicine
A trade name
of KO-IRBESAN
the International unlicensed name
Is not present
the Dosage form of the Tablet, film coated, 150 mg / 12.5 mg and 300 mg / 12.5 mg
Structure
One tablet contains
active agent irbesartan 150.00 mg or 300.00 mg
Hydrochlorthiazidum of 12.50 mg,
excipients: tselaktoza 80, cellulose microcrystalline PH 102, starch prezhelatinizirovanny 1500, sodium of a kroskarmelloz, aerosil 200, magnesium stearate, ferrous oxide red, ferrous oxide yellow,
structure of a cover: the gipromeloza, the titan dioxide, carmoisine Aluminium Lake (E 122), FD & C yellow #6 sanst yellow (E110), FD & C blue #2 Al Indigo carmine (E132), ferrous oxide yellow (E172), hydroxypropyl cellulose, water cleaned
the Description
of the Tablet, film coated pink color, an oval form, biconvex and from risky on one party (for a dosage of 150 mg / 12.5 mg).
Tablets, film coated pink color, oblong shape, biconvex and with profound risky on one party (for a dosage of 300 mg / 12.5 mg).
Pharmacotherapeutic group
the Drugs influencing a system renin-angiotensin.
Angiotensin II antagonists in a combination with diuretics.
Code of automatic telephone exchange C09DA04
Pharmacological
Pharmacokinetics Combined properties antihypertensive drug. After intake irbesartan and Hydrochlorthiazidum are well soaked up. The absolute bioavailability of an irbesartan is about 60 - 80%. The maximum concentration of an irbesartan in blood plasma is reached in 1.5 - 2 h, reaching up to 80 - 85%. The concomitant use of food significantly does not affect bioavailability of an irbesartan. Linking of an irbesartan with proteins of blood plasma makes about 96% at insignificant linking with cellular components of blood. Elimination half-life of an irbesartan in a terminal phase makes 11 - 15 h. Equilibrium concentration of an irbesartan in blood plasma is reached within 3 days since the beginning of use of drug at reception of 1 times a day. The linear dependence on a dose in the range of doses from 10 to 600 mg is characteristic of pharmacokinetic indicators of an irbesartan. Weaker, than proportional, increase at absorption was observed at intake in doses more than 600 mg. At repeated receptions of 1 times a day the limited accumulation of an irbesartan in blood plasma is noted (& lt, 20%). The general clearance of an irbesartan of an organism and renal clearance is made by 157 - 176 and 3 - 3.5 ml/min. respectively. Irbesartan is metabolized in a liver by conjugation with formation of a glucuronide and oxidation. The main metabolite circulating in blood is the irbesartana a glucuronide (about 6%). Irbesartan is oxidized mainly with participation of CYP P450 cytochrome 2C9 while the isoenzyme of CYP 3A4 is of little importance. Irbesartan and his metabolites are brought out of an organism both with bile, and with urine. About 20% are removed with urine, and the rest - with a stake. Less than 2% of the accepted dose are allocated with urine in the form of not changed irbesartan.
Hydrochlorthiazidum at oral administration is pretty fast soaked up from digestive tract. The bioavailability is about 60 - 80%. During 24 h in not changed look, at least, 61% of an oral dose are removed. Hydrochlorthiazidum is not metabolized, and quickly emitted with kidneys. Gets through a placental barrier, but does not get through a blood-brain barrier (GEB). The diuretic effect develops in 2 hours after oral administration, reaches a maximum approximately by the 4th o'clock and lasts from 5.6 to 14.8 hours. Elimination half-life makes of 6 - 10 o'clock.
The pharmacodynamics
of KO-IRBESAN is a combination irbesartana-the selection antagonist of receptors of angiotensin ІІ (AT1 type) and Hydrochlorthiazidum - diuretic of a class of tiazid. At combined use of an irbesartan and Hydrochlorthiazidum the antihypertensive effect increases. Irbesartan blocks all physiologically significant effects of angiotensin II which are implemented through AT1 receptors irrespective of a source or a way of synthesis of angiotensin II. Specific antagonistic action of an irbesartan concerning receptors of angiotensin II (AT1) leads to increase in concentration of renin and angiotensin II in blood plasma and to decrease in concentration of Aldosteronum in blood plasma. When using drug in the recommended dose the concentration of potassium ions in blood serum significantly does not change. Does not inhibit APF (a kininaz of II - the enzyme which is responsible for formation of angiotensin II), causes metabolic degradation of bradykinin with formation of inactive metabolites. Manifestation of effect does not require metabolic activation. CO-Irbesan lowers the arterial blood pressure at the minimum change of the heart rate (HR). Decrease in the ABP at reception of 1 times a day has dose-dependent character, with a tendency to an exit to the plateau when assigning in doses more than 300 mg. Purpose of 1 tablet of 1 times a day causes decrease in the ABP during 24 h after administration of drug (in a prone position or sitting). The maximum decrease in the ABP level is reached in 3 - 6 h after administration of drug, the hypotensive effect remains at least during 24 h. In 24 h after reception in the recommended doses the decrease in the ABP is 50 - 70% in comparison with the maximum level of decrease in diastolic and systolic arterial blood pressure. Administration of drug of KO-IRBESAN in a dosage of 150 mg/12.5 mg of 1 times a day is accorded by the effect similar to that which is reached at division of this daily dose into 2 receptions. At regular use during 12 weeks the effect gains stability and in 46 weeks reaches a maximum. The hypotensive effect remains at long-term treatment. After the termination of treatment of the ABP gradually returns to initial level.
Hydrochlorthiazidum affects mechanisms of a reabsorption of electrolytes in renal tubules, directly strengthening discharge of ions of sodium and chlorine in approximately identical quantities. Indirectly, as a result of diuretic activity of Hydrochlorthiazidum, blood plasma volume decreases therefore the activity of renin increases in plasma, secretion of Aldosteronum increases, loss of potassium increases in urine and potassium content in blood serum decreases. The hydrochlorothiazide is thiazide diuretic of tiazid and is used at treatment of hypertensia and hypostases. Thiazide diuretics influence renal mechanisms of a reabsorption of electrolytes, increasing excretion of sodium and chlorine
Dov in approximately equivalent quantities, brake a sodium reabsorption. The mechanism of action is connected with influence on distal tubules of nephron where the reabsorption of ions of sodium, chlorine and water decreases. Excretion of potassium ions, magnesium and bicarbonate increase, calcium ions are late in an organism. The hydrochlorothiazide reduces plasma volume, increasing activity of renin in plasma and secretion of Aldosteronum, with the subsequent increase in content of potassium in urine and decrease in its content in blood serum. By means of blockade the combined use of an irbesartan leads system renin-angiotensin-aldosteronovoy to prevention of loss of potassium in the blood serum caused by this diuretic.
At the same time diuretics of group of tiazid render hypotensive effect by means of decrease in peripheric resistance.
At combined use Hydrochlorthiazidum not only strengthens, but also angiotensin II extends antihypertensive action of blockers of receptors. Besides, combined use of blockers of receptors and thiazide diuretic drugs leads angiotensin II to noticeable decrease in the ABP at patients both with high, and with low activity of renin, and the response to this combination therapy makes about 80% and above.
Indications - essetsialny arterial hypertension - arterial hypertension symptomatic, including at patients with diseases of kidneys and diabetes ІІ type (as a part of antihypertensive therapy)
the Route of administration and doses
Adult Patients from whom it is not possible to achieve adequate control of arterial blood pressure at monotherapy irbesartany or Hydrochlorthiazidum, can be transferred to administration of drug of KO IRBESAN.
To patients with the arterial blood pressure (ABP) more than 160/100 mm Hg at a combination to diabetes or/and chronic kidney disease the combination therapy can be appointed on start of treatment from purpose of 1 tablet KO-IRBESAN in a dosage of 150 mg/12.5 mg of 1 times a day which, if necessary, is raised subsequently to 300 mg / 12.5мг 1 time in day.
An initial and maintenance dose - 1 tablet TO - IRBESAN in a dosage of 150 mg / 12.5 mg a day regardless of meal. The effective dose of Hydrochlorthiazidum usually makes 12.5 mg/days.
KO-IRBESAN in a dosage of 150 mg / 12.5 usually provides mg of 1 times a day optimum 24 - hour control of the ABP. At insufficient efficiency of a dose of 150 mg / 12.5 mg of drug KO-IRBESAN of 1 times a day appoint 300 mg / 12.5мг drug.
Use of drug in doses of more than 300 mg irbesartana/25 is not recommended to mg of a hydrochlorothiazide of 1 times/days.
Side effects
- dizziness, orthostatic dizziness, the general weakness
- orthostatic hypotension
- nausea, vomiting, the dispeptic phenomena, heartburn
- cough, rhinitis, pharyngitis
- musculoskeletal pains (myalgias, ossalgiya, thorax pains)
- increase in the KFK level in blood plasma, a hyperpotassemia
Seldom
- a headache, a ring in ears, fatigue, a condition of alarm / excite
pave
- tachycardia, rushes of blood
- rash, urticaria, a Quincke's disease
- disturbance of flavoring feelings
- an abnormal liver function, hepatitis
- an abdominal cavity pains
- infections of urinary tract, a renal failure (in edinich
ny cases - a renal failure at patients of group of the increased
risk)
- decrease in level of hemoglobin
of the Contraindication
- hypersensitivity to drug components
- primary hyper aldosteronism
- a hypovolemia (including against the background of high doses of diuretics)
- an anury
- pregnancy and the period of a lactation
- children's and teenage age up to 18 years
Medicinal interaction At simultaneous use of KO-IRBESAN with: - thiazide diuretics, the antihypertensive effect of an irbesartan amplifies. the previous treatment by diuretics in high doses can lead to dehydration and increases risk of developing symptomatic hypotension, - with the kaliysberegayushchy diuretics and drugs of potassium, substitutes of table salt and other drugs increasing potassium level in blood serum (for example heparin), risk of development of a hyperpotassemia increases,
- other antihypertensive drugs, the hypotensive effect amplifies, we will combine with beta blockers, blockers of calcium channels, digoxin, Cimetidinum, - lithium drugs, increase in concentration of lithium in blood plasma is possible and development of its toxic effects therefore such combination is not recommended if such combination is necessary, is recommended to carry out careful control of level of lithium in blood serum, - warfarin, tolbutamide (CYP substrates 2C9) and also nifedipine (CYP inhibitor 2C9), noticeable pharmacokinetic or pharmakodinamichesky interactions are not revealed, KO-IRBESAN can be combined safely with blockers ‑ adrenoceptors, antagonists of calcium of long action and thiazide diuretics. TO - IRBESAN in a dosage of 150 mg / 12.5мг does not influence digoxin pharmacokinetics. Operation of inductors CYP 2C9, such as rifampicin, is unknown.
Special instructions At patients with dehydration and at patients with a hyponatremia who resulted from intensive treatment by diuretics of diarrhea, vomiting or limited consumption of salt, symptomatic hypotension, especially after reception of the first dose of drug can develop. The hypovolemia and a hyponatremia have to be eliminated prior to use of drug KO-IRBESAN. Patients with a bilateral stenosis of renal arteries or a stenosis of an artery of the only functioning kidney who take other drugs influencing renin - angiotensin - an aldosteronovy system treat group of the increased risk concerning development of heavy arterial hypotension or a renal failure. Though such cases for drug TO - IRBESAN are not described, the similar effect can be expected when using antagonists of receptors of angiotensin II. As well as in case of prescribing of any other hypotensive drug, excessive decrease in the ABP at patients with an ischemic cardiopathy or an ischemic heart disease can lead to a myocardial infarction or a stroke. As well as when using other drugs influencing renin-angio-tenzin-aldosteronovuyu a system during the KO-IRBESAN drug treatment the hyperpotassemia, especially in the presence of a renal failure, the proteinuria caused by a diabetic nephropathy and/or heart diseases can be observed. For identification of a hyperpotassemia during drug treatment the monitoring of level of potassium and creatinine in blood serum is recommended. As well as when using other vazodilatator, it is necessary to take special precautionary measures for patients with an aortal or mitral stenosis or obstructive hypertrophic kardiomio
a patiya. At patients, the vascular tone and which function of kidneys depend mainly on activity system renin-angiotensin-aldosteronovoy (for example at severe forms of stagnant heart failure or diseases of kidneys, including a renal artery stenosis), treatment by APF inhibitors or antagonists of receptors of angiotensin ІІ can cause acute arterial hypotension, an azotemia, an oliguria and in rare instances - OPN. There are no clinical data on use of drug KO-IRBESAN for the patients who transferred transplantation of kidneys. At establishment of pregnancy of KO-IRBESAN it is necessary to cancel as soon as possible. For detection of malformations it is recommended to carry out ultrasonography of a fruit. It is unknown whether it is excreted irbesartan in breast milk. Use in pediatrics Safety and efficiency of drug at children are not established. The feature of influence of medicine on ability to run vehicles and potentially dangerous mechanisms Should pay attention to a possibility of development of side effects (for example, dizzinesses) which can affect ability to run the vehicle or potentially dangerous mechanisms. Patients need to be warned about danger of performance of the work requiring special attention and speed of psychomotor reactions before disappearance of these side effects.
The overdose
to Develop