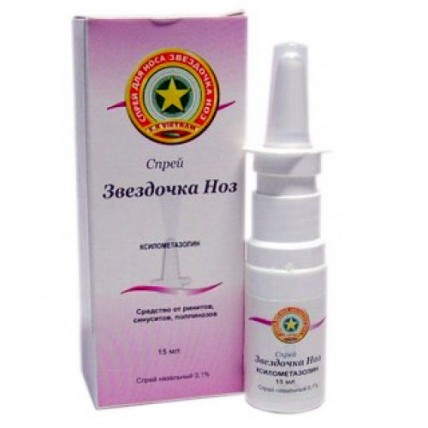
Gold star 0.1%, 15 ml nasal spray
- $5.80
The instruction for medical use
of GOLD STAR SPRAY medicine
the Trade name
Gold Star Spray
the International unlicensed
name Xylomethazolinum Dosage Form Spray of Nasal 0.1% 15 Ml Structure
1 bottle contains
active agent - xylomethazolinum a hydrochloride of 15.0 mg
excipients: sodium dihydrophosphate, sodium hydrophosphate, sodium chloride, a benzalkoniya chloride, water purified.
Description
Transparent colourless liquid.
Pharmacotherapeutic group
Nasal drugs. Antikongestanta. Antikongestanta and other nasal drugs for topical administration. Xylomethazolinum.
ATH R01AA07 code
the Pharmacological
Pharmacokinetics At properties topical administration is practically not absorbed, concentration in plasma are so small that they cannot be determined by modern analytical methods.
The pharmacodynamics
Alpha adrenostimulyator, narrows blood vessels of a mucous membrane of a nasal cavity, eliminating hypostasis and hyperaemia of a mucous membrane. Facilitates nasal breath in rhinitises. Action comes in a few minutes and continues within several hours.
Indications
- acute allergic rhinitis,
- ORZ with the rhinitis phenomena,
- sinusitis,
- a pollinosis,
- average otitis (for reduction of hypostasis of a mucous membrane of a nasopharynx),
- training of the patient for diagnostic manipulations in the nasal courses.
Route of administration and
Intranazalno's doses. For adults and children 12 years are more senior: On 3 injections in each nasal course (if necessary it is possible to repeat), it is not necessary to apply more than 3 times a day. Drug is used no more than 7 days.
Side effects
At frequent and/or prolonged use are possible:
- irritation and or dryness of a mucous membrane of a nasopharynx, burning, pricking, sneezing, hypersecretions.
Seldom:
- the rhinedema,
- heartbeat, tachycardia, arrhythmia
- a headache, increase in arterial blood pressure
- vomiting
- insomnia, a depression (at prolonged use of high doses)
- a disorder of vision
At emergence of symptoms to stop use of drug and to see a doctor.
Contraindications
- hypersensitivity to drug
- arterial hypertension, tachycardia
- the profound atherosclerosis
- glaucoma
- atrophic rhinitis
- surgical interventions on a meninx (in the anamnesis)
- children's age up to 12 years
- a thyrotoxicosis
- pregnancy
- use of monoamine oxidase inhibitors and tricyclic antidepressants.
Not to use medicinal interactions along with MAO inhibitors and tricyclic antidepressants.
Special instructions
to take at an ischemic heart disease (stenocardia), a prostate hyperplasia, diabetes with caution.
In chronic rhinitis drug is not appointed.
Before use remove a protective cap of the pump doser. Easy pressing of fingers make 2 trial injections (not in a nose), and then inject quantity of the doses registered by the doctor into a nose. After use always close the pump doser a protective cap.
Pregnancy and a lactation
At pregnancy the administration of drug is contraindicated, in the period of a lactation to take drug with caution.
Feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
Drug does not reduce ability to run transport and to work with mechanisms.
Overdose
Symptoms: a headache, dryness in a nose, nausea, a depression, increase in arterial blood pressure, a short-term disorder of vision.
Treatment: it is necessary to stop use of drug and to apply drugs antagonists: sympatholytics and α-adrenoblockers (phentolamine, Tropaphenum), symptomatic therapy.
At accidental ingestion of drug (is more often at children) the tachycardia, arrhythmia, increase in arterial blood pressure, drowsiness, confusion of consciousness, a depression of breath or irregular breath is noted.
Symptomatic treatment.
A form of release and packing
On 15 ml of drug in a plastic bottle with the spray and with a protective cap from polyethylene.
On a bottle paste the label self-adhesive.
On 1 bottle with the spray together with the instruction for medical use in the state and Russian languages place in a pack from cardboard.
To Store storage conditions in the dry place protected from light at a temperature from 15 ºС up to 25 ºС
to Store out of children's reach!
Period of storage
3 years.
Not to apply after the expiration date specified on packing.
Prescription status
Without prescription.
Danafa Pharmaceutical Dzhoynt Stock Company producer
Vietnam, Da Nang, Tkhan Tkhe's region, Zung Si St. of Tkhan Tkhe, 253.
Owner of the registration certificate
of Danafa Pharmaceutical Dzhoynt Stock Company
Vietnam, Da Nang, Tkhan Tkhe's region, Zung Si St. of Tkhan Tkhe, 253.
The address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods)
Address: Republic of Kazakhstan, Almaty, Itev St. 46-18
Ph. / fax: +7 (727) 395 91 13
Mobile phone number: +7 (701) 718 25 92
E-mail address: layka16@mail.ru
of GOLD STAR SPRAY medicine
the Trade name
Gold Star Spray
the International unlicensed
name Xylomethazolinum Dosage Form Spray of Nasal 0.1% 15 Ml Structure
1 bottle contains
active agent - xylomethazolinum a hydrochloride of 15.0 mg
excipients: sodium dihydrophosphate, sodium hydrophosphate, sodium chloride, a benzalkoniya chloride, water purified.
Description
Transparent colourless liquid.
Pharmacotherapeutic group
Nasal drugs. Antikongestanta. Antikongestanta and other nasal drugs for topical administration. Xylomethazolinum.
ATH R01AA07 code
the Pharmacological
Pharmacokinetics At properties topical administration is practically not absorbed, concentration in plasma are so small that they cannot be determined by modern analytical methods.
The pharmacodynamics
Alpha adrenostimulyator, narrows blood vessels of a mucous membrane of a nasal cavity, eliminating hypostasis and hyperaemia of a mucous membrane. Facilitates nasal breath in rhinitises. Action comes in a few minutes and continues within several hours.
Indications
- acute allergic rhinitis,
- ORZ with the rhinitis phenomena,
- sinusitis,
- a pollinosis,
- average otitis (for reduction of hypostasis of a mucous membrane of a nasopharynx),
- training of the patient for diagnostic manipulations in the nasal courses.
Route of administration and
Intranazalno's doses. For adults and children 12 years are more senior: On 3 injections in each nasal course (if necessary it is possible to repeat), it is not necessary to apply more than 3 times a day. Drug is used no more than 7 days.
Side effects
At frequent and/or prolonged use are possible:
- irritation and or dryness of a mucous membrane of a nasopharynx, burning, pricking, sneezing, hypersecretions.
Seldom:
- the rhinedema,
- heartbeat, tachycardia, arrhythmia
- a headache, increase in arterial blood pressure
- vomiting
- insomnia, a depression (at prolonged use of high doses)
- a disorder of vision
At emergence of symptoms to stop use of drug and to see a doctor.
Contraindications
- hypersensitivity to drug
- arterial hypertension, tachycardia
- the profound atherosclerosis
- glaucoma
- atrophic rhinitis
- surgical interventions on a meninx (in the anamnesis)
- children's age up to 12 years
- a thyrotoxicosis
- pregnancy
- use of monoamine oxidase inhibitors and tricyclic antidepressants.
Not to use medicinal interactions along with MAO inhibitors and tricyclic antidepressants.
Special instructions
to take at an ischemic heart disease (stenocardia), a prostate hyperplasia, diabetes with caution.
In chronic rhinitis drug is not appointed.
Before use remove a protective cap of the pump doser. Easy pressing of fingers make 2 trial injections (not in a nose), and then inject quantity of the doses registered by the doctor into a nose. After use always close the pump doser a protective cap.
Pregnancy and a lactation
At pregnancy the administration of drug is contraindicated, in the period of a lactation to take drug with caution.
Feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
Drug does not reduce ability to run transport and to work with mechanisms.
Overdose
Symptoms: a headache, dryness in a nose, nausea, a depression, increase in arterial blood pressure, a short-term disorder of vision.
Treatment: it is necessary to stop use of drug and to apply drugs antagonists: sympatholytics and α-adrenoblockers (phentolamine, Tropaphenum), symptomatic therapy.
At accidental ingestion of drug (is more often at children) the tachycardia, arrhythmia, increase in arterial blood pressure, drowsiness, confusion of consciousness, a depression of breath or irregular breath is noted.
Symptomatic treatment.
A form of release and packing
On 15 ml of drug in a plastic bottle with the spray and with a protective cap from polyethylene.
On a bottle paste the label self-adhesive.
On 1 bottle with the spray together with the instruction for medical use in the state and Russian languages place in a pack from cardboard.
To Store storage conditions in the dry place protected from light at a temperature from 15 ºС up to 25 ºС
to Store out of children's reach!
Period of storage
3 years.
Not to apply after the expiration date specified on packing.
Prescription status
Without prescription.
Danafa Pharmaceutical Dzhoynt Stock Company producer
Vietnam, Da Nang, Tkhan Tkhe's region, Zung Si St. of Tkhan Tkhe, 253.
Owner of the registration certificate
of Danafa Pharmaceutical Dzhoynt Stock Company
Vietnam, Da Nang, Tkhan Tkhe's region, Zung Si St. of Tkhan Tkhe, 253.
The address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods)
Address: Republic of Kazakhstan, Almaty, Itev St. 46-18
Ph. / fax: +7 (727) 395 91 13
Mobile phone number: +7 (701) 718 25 92
E-mail address: layka16@mail.ru