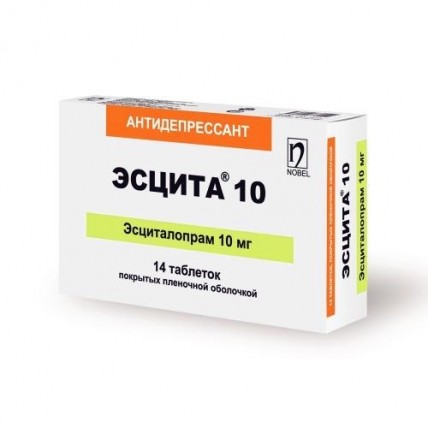
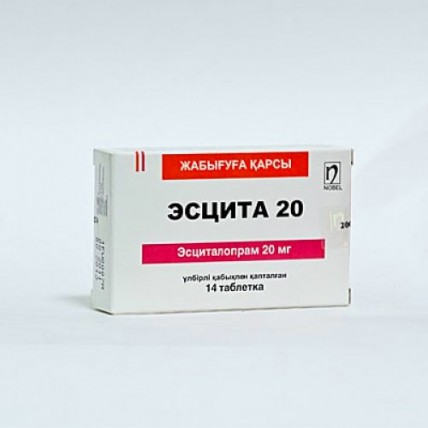
ESCITA® (Escitalopram) 10/20 mg
- $18.00
Composition
One tablet contains:
Active ingredient - escitalopram oxalate 12.775 mg or 25.55 mg (equivalent to 10 mg and 20 mg of escitalopram, respectively).
Excipients: crospovidone (collidon VA 64), lactose monohydrate, maize starch, microcrystalline cellulose PH 102, sodium croscarmellose, magnesium stearate.
Sepifilm LP 770 coating composition: hydroxypropyl methylcellulose (E 464), microcrystalline cellulose (E 460), stearic acid (E 570), titanium dioxide (E 171).
Description of ESCITA® Tablets ESCITA® 10: 10 mg film-coated tablets, white, oval, biconvex, with a scoreline on one side and "10" marking on the other. ESCITA® 20: 20 mg film-coated tablets, white, oval, biconvex, with a scoreline on one side and "20" marking on the other.
Pharmacological Properties
Pharmacokinetics
Absorption is not affected by food intake. After oral administration, the average time to reach maximum concentration (Cmax) in the blood plasma is approximately 4 hours. The bioavailability of escitalopram is about 80%.
The binding of escitalopram and its major metabolites to plasma proteins is less than 80%.
Escitalopram is metabolized in the liver to demethylated and didemethylated metabolites, which are pharmacologically active. The kinetics of escitalopram is linear. Steady-state concentration (Css) is achieved in approximately 1 week.
The elimination half-life (T1/2) after multiple doses is about 30 hours. Clearance after oral administration is approximately 0.6 L/min. The major metabolites of escitalopram have a longer half-life. Escitalopram and its major metabolites are eliminated by the liver (metabolic pathway) and the kidneys. Most of it is excreted as metabolites in the urine.
Elderly Patients (>65 years) Escitalopram is eliminated more slowly in elderly patients compared to younger individuals. Systemic exposure (AUC) is approximately 50% higher in elderly patients compared to young healthy volunteers.
Impaired Liver Function In patients with mild or moderate impairment of liver function, the elimination half-life of escitalopram is approximately twice as long, and exposure is approximately 60% higher than in people with normal liver function.
Impaired Kidney Function For escitalopram, a longer elimination half-life and a slight increase in exposure were observed in patients with impaired kidney function (CLcr 10-53 ml/min). Concentrations of metabolites in plasma were not studied but may be elevated.
Pharmacodynamics
ESCITA® is an antidepressant, a selective serotonin reuptake inhibitor (SSRI). Inhibition of serotonin reuptake leads to an increase in the concentration of this neurotransmitter in the synaptic cleft, enhancing and prolonging its action on postsynaptic receptor sites.
Escitalopram is practically not associated with several receptors, including serotonin 5-HT1A, 5-HT2 receptors, dopamine D1 and D2 receptors, α1, α2, β-adrenergic receptors, histamine H1 receptors, muscarinic receptors, benzodiazepine, and opioid receptors.
The mechanism of the antidepressant action of escitalopram is linked to the enhancement of serotonergic activity in the central nervous system (CNS) due to the inhibition of neuronal serotonin reuptake (5-HT). Escitalopram is a highly selective serotonin reuptake inhibitor with minimal impact on the reuptake of norepinephrine and dopamine. Escitalopram (S-enantiomer) is a much more potent inhibitor (100 times) of the serotonin transporter protein involved in serotonin reuptake than the racemic form of citalopram - R-enantiomer. Escitalopram also does not bind or has very weak binding ability to ion channels, including Na+, K+, Cl-, Ca++ channels.
Indications for Use
- Depression
- Panic disorders with/without agoraphobia
- Social anxiety (social phobia)
- Generalized anxiety disorders
- Obsessive-compulsive disorders
Dosage and Administration
The medication is prescribed once daily regardless of meals.
Regarding the safety of a daily dose exceeding 20 mg of escitalopram, there is no available data.
Major depressive episodes: The usual starting dose of the medication is 10 mg/day. Depending on the individual patient's response, the dose may be increased to a maximum of 20 mg/day.
The antidepressant effect typically develops within 2-4 weeks after starting treatment. After the disappearance of depressive symptoms, therapy should be continued for at least another 6 months to consolidate the achieved effect.
Panic disorders with/without agoraphobia: During the first week of treatment, a dose of 5 mg/day is recommended, with subsequent titration to 10 mg/day. Depending on the individual patient's response, the dose may be increased to a maximum of 20 mg/day.
The maximum therapeutic effect is usually achieved approximately 3 months after the start of treatment. Therapy typically lasts for several months.
Social anxiety (social phobia): The usual starting dose of the medication is 10 mg/day. Depending on the individual patient's response, the dose may be increased to a maximum of 20 mg/day.
Anxiety states associated with social status are considered chronic conditions, and treatment with the medication for 12 weeks is recommended to enhance the response.
Generalized anxiety disorders: The initial dose of the medication is 10 mg/day. Depending on the individual patient's response, the dose may be increased to a maximum of 20 mg/day.
Generalized anxiety disorders are chronic conditions, and treatment with the medication for 12 weeks is recommended to enhance the response. The benefit-to-dose ratio of the medication should be reevaluated at regular intervals during treatment.
Obsessive-compulsive disorders: The initial dose of the medication is 10 mg/day. Depending on the individual patient's response, the dose may be increased to a maximum of 20 mg/day.
Obsessive-compulsive disorders are chronic conditions, and treatment of such patients should continue for an adequate period until complete recovery.
Elderly patients (over 65 years old) A half of the usually recommended dose (5 mg/day) and a lower maximum dose (10 mg/day) are prescribed.
The efficacy of the medication in elderly patients with social phobia has not been studied.
For patients with mild to moderate renal impairment, dose adjustment is not required. A recommended initial dose for two weeks is 5 mg/day. Patients with severe renal impairment (creatinine clearance <30 ml/min) should receive the medication with caution.
For patients with impaired liver function, the recommended initial dose for the first 2 weeks of treatment is 5 mg/day. Depending on the individual response to treatment, the dose may be increased to 10 mg/day. Patients with severe liver impairment should receive the medication with caution.
In cases of reduced CYP2C19 enzyme activity, the recommended initial dose for the first 2 weeks of treatment is 5 mg/day. Depending on the individual response to treatment, the dose may be increased to 10 mg/day.
When discontinuing treatment with the medication, the dose should be gradually reduced over 1-2 weeks to avoid withdrawal syndrome.
Side Effects
Common:
- Nausea, decreased appetite, diarrhea, constipation, abdominal pain
- Insomnia or drowsiness, dizziness, weakness
- Increased sweating, hyperthermia
- Reduced libido, impotence, ejaculation disorders, anorgasmia (in women)
Rare:
- Taste disturbances
- Vomiting, dry mouth, changes in liver function tests
- Orthostatic hypotension
- Hyponatremia
- Inadequate ADH secretion, galactorrhea
- Anaphylactic reactions, angioedema
- Skin rash, itching, ecchymosis, purpura
- Sinusitis
- Joint pain, muscle pain, urinary retention
Possible:
- Visual disturbances, seizures, tremor, motor disturbances, serotonin syndrome, hallucinations, mania, confusion, agitation, personality splitting with suicidal tendencies, panic attacks, increased irritability.
Upon abrupt discontinuation of the medication after prolonged use, withdrawal reactions such as dizziness, headaches, and nausea may occur. These reactions are generally mild and of limited duration.
Side effects most commonly occur in the first or second week of treatment and usually become less intense and less frequent with continued therapy.
Contraindications
- The use of the drug Escita® is not recommended in the following cases:
- High sensitivity to escitalopram or other components of the drug.
- Concurrent use with monoamine oxidase inhibitors (MAOIs).
- Concurrent use with pimozide.
- Pregnancy and lactation.
- Pediatric and adolescent patients under 18 years of age.
Drug Interactions:
Interactions when used concurrently with Escita®:
- With MAO inhibitors, as well as in the early use of MAO inhibitors by patients who have recently discontinued Escita®, serious adverse reactions may occur. In such cases, serotonin syndrome may develop.
- With serotonergic drugs (such as tramadol, sumatriptan, and other triptans), it may lead to the development of serotonin syndrome.
- Escita® may lower the seizure threshold, so caution is needed when prescribing Escita® along with other drugs that lower the seizure threshold.
- Escita® may enhance the action of lithium and tryptophan.
- With oral anticoagulants and drugs affecting blood clotting (such as atypical antipsychotics, phenothiazines, most tricyclic antidepressants, acetylsalicylic acid, NSAIDs, ticlopidine, and dipyridamole), there may be a disturbance in blood clotting. In such cases, careful monitoring of blood clotting is necessary at the beginning or end of Escita® therapy.
- Drugs inhibiting the CYP2C19 isoenzyme may increase the concentration of Escita® in the bloodstream. Caution should be exercised when using Escita® concurrently with such drugs (including omeprazole), and a reduction in the Escita® dose may be necessary.
- High doses of Escita® should be administered with caution along with high doses of cimetidine, which is a strong inhibitor of the CYP2D6, CYP3A4, and CYP1A2 isoenzymes.
- Simultaneous administration of Escita® and preparations containing St. John's Wort (Hypericum perforatum) may lead to an increased number of side effects.
- When alcohol is taken concurrently with Escita®, there is no pharmacodynamic or pharmacokinetic interaction, but as with other psychotropic drugs, simultaneous use of Escita® and alcohol is not recommended.
- Escita® is an inhibitor of the CYP2D6 isoenzyme. Caution should be exercised when concurrently prescribing escitalopram and drugs metabolized by this isoenzyme with a narrow therapeutic index, such as flecainide, propafenone, and metoprolol (in cases of use for heart failure), or drugs primarily metabolized by the CYP2D6 isoenzyme and acting on the central nervous system (e.g., antidepressants desipramine, clomipramine, nortriptyline, or antipsychotics risperidone, thioridazine, haloperidol). In these cases, a dose adjustment may be required.
- Simultaneous administration of Escita® with desipramine or metoprolol leads to a twofold increase in the concentration of the latter two drugs.
- Escita® may slightly inhibit the CYP2C19 isoenzyme. Therefore, caution is recommended when using Escita® concurrently with drugs metabolized with the involvement of this isoenzyme.
Special Precautions
- Escita® should not be prescribed simultaneously with MAO inhibitors. The drug can be administered 14 days after discontinuing treatment with irreversible MAO inhibitors and at least 1 day after discontinuing therapy with reversible MAO-A inhibitors (including moclobemide). At least 7 days should elapse after discontinuing Escita® before starting treatment with non-selective MAO inhibitors.
- Some patients with panic disorders may experience increased anxiety at the beginning of treatment with selective serotonin reuptake inhibitors (including Escita®). This paradoxical reaction typically disappears within 2 weeks of treatment. To reduce the likelihood of an anxiogenic effect, it is recommended to use the drug at low initial doses.
- Escita® should be discontinued in the event of seizures.
- The use of the drug is not recommended in patients with unstable epilepsy, and in patients with controlled seizures, careful monitoring is necessary. In case of an increase in the frequency of seizures, selective serotonin reuptake inhibitors, including Escita®, should be discontinued.
- Escita® should be used with caution in patients with a history of mania/hypomania. If a manic state develops, Escita® should be discontinued.
- When treating patients with diabetes with Escita®, there may be changes in blood glucose levels, requiring insulin dose adjustments and/or adjustments to oral hypoglycemic agents.
- The risk of suicide is associated with depression and may persist until a substantial improvement occurs, either spontaneously or as a result of treatment.
- Close observation is necessary for patients receiving antidepressant treatment, especially at the beginning, due to the possibility of clinical deterioration and/or the emergence of suicidal thoughts and behavior. This precaution should be exercised when treating other mental disorders accompanied by depressive episodes.
- Hyponatremia, possibly associated with impaired ADH secretion, rarely occurs with Escita® therapy and usually resolves upon discontinuation of treatment.
- Escita® should be used with caution in patients at risk of developing hyponatremia, including the elderly, patients with liver cirrhosis, and those taking medications capable of causing hyponatremia.
- When taking Escita®, skin hemorrhages (ecchymosis and purpura) may occur. Caution should be exercised when using Escita® in patients prone to bleeding or taking oral anticoagulants and drugs that affect blood clotting.
- Clinical experience with the combined use of Escita® and electroconvulsive therapy is limited; therefore, caution should be exercised in such cases.
- Simultaneous use of Escita® and alcohol is not recommended.
- Escita® should be used with caution in patients with severe renal impairment (creatinine clearance <30 mL/min).
Pregnancy:
- The use of SSRIs in the third trimester of pregnancy can negatively impact the psychophysical development of the newborn. Neurological disorders have been reported in newborns whose mothers took SSRIs up to delivery, including apnea, cyanosis, seizures, hypoglycemia, hypotonia, irritability, tremor, hypertonia, unstable temperature, constant crying, feeding difficulties, and poor sleep. These symptoms may indicate serotoninergic effects or withdrawal syndrome. If SSRIs are used during pregnancy, their discontinuation should not be abrupt; it should be done gradually.
Impact on Driving and Operating Machinery:
- Caution should be exercised when operating a vehicle or potentially hazardous machinery.
Overdose
Symptoms of overdose may include dizziness, tremor, emotional and motor agitation, drowsiness, loss of consciousness, seizures, tachycardia, ECG changes (ST segment and T wave alterations, QRS complex widening, QT interval prolongation), arrhythmias, respiratory depression, vomiting, rhabdomyolysis, metabolic acidosis, hypokalemia.
Treatment for overdose: There is no specific antidote. Gastric lavage, adequate oxygen therapy, monitoring of respiration and cardiovascular activity, and symptomatic treatment may be required.
Storage Conditions
- Store at temperatures not exceeding 25°C in a dry place, protected from light.
- Keep out of reach of children.
Shelf Life
- 3 years. Do not use after the expiration date.