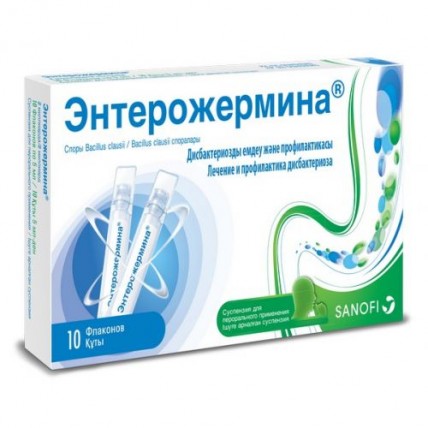
Enterozhermina® 2 billion / 5 ml 10s oral suspension
- $22.60
Sku:
ba12ce8fa390
Brand:
Sanofi SpA (Italy)
The instruction for medical use of ENTEROZhERMINA medicine the Trade name of Enterozhermina the International unlicensed name Is not present the Dosage form Suspension for oral administration 2 billion / 5 ml Structure of 5 ml of suspension contains active agent - spores of Bacillus clausii polyresistant to various chemotherapeutic drugs and antibiotics - 2 billion spores, excipient - the water purified. The description Whitish opalescent liquid with a specific smell. Pharmacotherapeutic group Digestive tract and metabolism. Antidiarrheal, intestinal anti-inflammatory/antimicrobial drugs. Anti-diarrheal microorganisms. The ATX A07FA code the Pharmacological Pharmacokinetics As properties active agent of the drug Enterozhermina are the spores of bacteria localized and operating only in a digestive tract gleam, pharmacokinetic researches were not conducted. The pharmacodynamics of Enterozhermina is the drug consisting of Bacillus clausii spores – the ordinary inhabitant of the intestines which do not have pathogenic effect. At oral administration Bacillus clausii spores, thanks to the high chemical and physical substances resistance, get through a barrier of gastric juice and in the intact state reach an intestinal path where they will be transformed to metabolic active vegetative cells. Thanks to action of Bacillus clausii the use of Enterozhermina medicine promotes restoration of the intestinal microflora broken as a result of influence of various factors. As Bacillus clausii is also capable to produce various vitamins, in particular vitamins of group B, Enterozhermina® contributes to normalization of disturbance of the vitamin balance in an organism caused by antibiotics and chemotherapy in general. Энтерожермина® allows to achieve the nonspecific antigenic and anti-toxic effect which is closely connected with metabolic action of Bacillus clausii. Besides, artificially caused heterogeneous resistance of high degree of Bacillus clausii to antibiotics provides a therapeutic basis for prevention of disturbances of intestinal microflora as a result of use of antibiotics, especially, of a broad spectrum of activity, or for recovery of balance of intestinal microflora. Due to this resistance to antibiotics, Enterozhermina can be applied in an interval between two doses of antibiotics. Drug has stability concerning penicillin (if not in a combination with inhibitors beta lactamelements), cephalosporins (partial stability in most cases), tetracyclines, macroleads, aminoglycosides (except for gentamycin and amikacin), chloramphenicol, a tiamfenikol, lincomycin, clindamycin, an isoniazid, Cycloserinum, novobiocin, rifampicin, Acidum nalidixicum and pipemidovy acid (intermediate stability) and metronidazole. Indications - treatment and prevention of disturbances of intestinal microflora and the subsequent endogenous disvitaminoz - auxiliary therapy for restoration of the intestinal microflora changed in the result of treatment with antibiotics or chemotherapy - acute or chronic gastrointestinal disorders at adults and children, including chest age, connected with poisoning or with intestinal dysbacteriosis (dismikrobiozy) and disvitaminozy the Route of administration and doses the Adult: 2-3 bottles a day. To children (including chest age): 1-2 bottles a day. Duration of treatment is defined individually by the doctor. The following duration of treatment depending on indications is recommended: - treatment of disturbances of intestinal microflora and subsequent endogenous disvitaminoz: in acute diarrhea of 5-7 days, in chronic diarrhea up to 30 days, - prevention of disturbances of intestinal microflora and the subsequent endogenous disvitaminoz during antibacterial therapy: during treatment by antibiotics and up to 1 week after the end of treatment by antibiotics, - auxiliary treatment for restoration of the intestinal microflora which changed during treatment by antibiotics or chemotherapy: during treatment by antibiotics and up to 1 week after the end of treatment by antibiotics, - treatment of acute gastrointestinal disorders (including infectious) at adults and children, including chest age, connected with poisoning or with intestinal dysbacteriosis (dismikrobiozy) and disvitaminozy: acute diarrhea of 5-7 days, - treatment of chronic gastrointestinal disorders (including infectious) at adults and children, including chest age, connected with poisoning or with intestinal dysbacteriosis (dismikrobiozy) and disvitaminozy: chronic diarrhea up to 30 days. Route of administration: Contents of bottles are accepted without cultivation or parted in water or other drinks (for example, in milk, tea, orange juice). This drug is intended only for intake. Not to enter parenterally and not to apply in a different way! Medicine has to be used in order to avoid suspension pollution Side effects at once Frequency is unknown - it is important to reaction of hypersensitivity (such as rash, urticaria and Quincke's disease) to report the Message about the suspected side reactions about the suspected side reactions during the post-registration period of use of medicine. It allows to conduct continuous monitoring of a ratio advantage/risk of drug. Experts in the field of health care are obliged to report about any cases of the suspected side reactions through national reporting system. Contraindications - hypersensitivity to active agent or components of drug Medicinal interactions are not established Special instructions Perhaps existence of visible inclusions in drug Enterozhermina® bottles that is caused by units of spores Bacillus clausii, it does not mean that drug underwent changes. It is necessary to stir up a bottle before use. This drug is intended only for oral administration! Injection or any other way of introduction in connection with high risk of heavy anaphylactic reactions, such as acute anaphylaxis is forbidden. During therapy by antibiotics the drug should be taken between two consecutive receptions of a dose of an antibiotic. In case the clinical state worsened or improvements did not come in 2-3 days of treatment and at emergence of the following symptoms: a heat, vomitings, blood or slime in Calais, strong thirst, dryness in a mouth, the patient has to see the attending physician. Pregnancy and the period of a lactation Intake of the IS CONTRAINDICATED NE this medicine during pregnancy and in the period of a lactation. Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms Drug does not affect ability to run the vehicle or moving mechanisms. Overdose Until now it was not reported about clinical manifestations of overdose. The form of release and packing On 5 ml of drug spill in bottles polyethylene. On bottles self-adhesive paste labels. On 10 bottles together with the instruction for medical use in the state and Russian languages place in a cardboard pack. To Store storage conditions at a temperature not above 30os. To store out of children's reach! 2 years not to apply a period of storage after an expiration date. Prescription status Without prescription the Producer/owner of the registration certificate of Sanofi S.P.A., Italy VIALE EUROPA, 11 ORIGGIO (VA) ITALIA the Name, the address and a contact information (phone, the fax, e-mail) of the organization in the territory of the Republic of Kazakhstan, the accepting claim (offer) on quality of medicines from consumers of Sanofi-aventis Kazakhstan LLP Republic of Kazakhstan, 050013, Almaty, Nazarbayev Ave 187 of B phone number: +7 (727) 244-50-96 fax: +7 (727) 258-25-96 e-mail: quality.info@sanofi.com the Name, the address and a contact information (phone, the fax, e-mail) of the organization in the territory of the Republic of Kazakhstan responsible for post-registration observation of safety of medicine of Sanofi-aventis Kazakhstan LLP Republic of Kazakhstan, 050013, Almaty, Nazarbayev Ave of 187 B phone number: +7 (727) 244-50-96 fax: +7 (727) 258-25-96 e-mail:
Kazakhstan.Pharmacovigilance@sanofi.com
Kazakhstan.Pharmacovigilance@sanofi.com