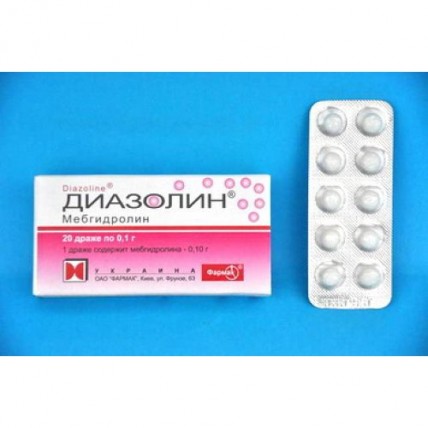
Diazolin 100 mg 20s dragee
- $3.30
Out Of Stock
The instruction for medical use
of DIAZOLINUM medicine
the Trade name
Diazolinum
the International unlicensed
name Mebgidrolin Lekarstvennaya a form
of the Dragee of 0.05 and 0.1 g
Structure
One dragee contains
active agent - a mebgidrolin in terms of 100% dry matter of-0.05 g or 0.1 g,
excipients: sucrose, starch syrup, talc, wax yellow, sunflower oil.
Description
of the Dragee of white color. At cross section two layers are visible. The dragee has to have the correct spherical shape. The surface of a dragee has to be equal, smooth and uniform
Pharmacotherapeutic group in color
Antihistamines for system use. Mebgidrolin
ATX R06A X15 Code
the Pharmacological
Pharmacokinetics Quickly properties is soaked up from a digestive tract. The bioavailability fluctuates within 40-60%. The therapeutic effect develops 15-30 minutes later, the maximum action is observed in 1-2 hours. Duration of effect can reach 2 days. Drug practically does not get through a blood-brain barrier, is metabolized in a liver by methylation, induces liver enzymes, is removed from an organism by kidneys.
The pharmacodynamics
Mebgidrolin belongs to antihistaminic drugs, is a blocker of Hl-receptors of a histamine. Mebgidrolin weakens spazmogenny effect of a histamine concerning unstriated muscles of bronchial tubes, intestines and also its influence on permeability of vessels. Unlike antihistaminic drugs of the first generation (Dimedrol, Suprastinum) has less significant sedative and somnolent effect. Possesses slight m-holinoblokiruyushchimi and the anesthetizing properties.
Indications
- prevention and treatment of seasonal and allergic rhinitis
- a pollinosis
- urticaria
- food and medicinal allergy
- skin reactions after stings of insects
- a dermatosis which is followed by a skin itching (eczema, neurodermatitis)
the Route of administration and doses
appoint Diazolinum inside, after a meal, to adults and children from 12 years on 0.1-0.2 g 1-2 times a day. The maximum doses for adults: single – 0.3 g, daily – 0.6 g.
To children of 5-12 years appoint 0.05 g 1-3 times a day, children are 3-5 years old – on 0.05 g 1-2 times a day. Duration of treatment is determined by the doctor depending on the nature of a disease, clinical effect and tolerance of drug.
Side effects
- the irritation of mucous membranes of digestive tract which are sometimes shown by the dispepsichesky phenomena (heartburn, nausea, pains in epigastric area)
- dizziness, paresthesias, increased fatigue, drowsiness, illegibility of visual perception, delay of speed of reactions, a tremor, uneasiness (night)
- dryness in a mouth, urination disturbances, allergic reactions
- is possible emergence of a granulocytopenia and an agranulocytosis
At children paradoxical reactions are sometimes observed: hyperexcitability, tremor, sleep disorders, irritability.
In isolated cases in the post-registration period the following side reactions were noted: headache, itching, rashes, urticaria, Quincke's edema.
Contraindications
- hypersensitivity to drug components
- a peptic ulcer of a stomach and duodenum during aggravation
- inflammatory diseases of digestive tract
- a pylorostenosis
- a prostate hyperplasia
- closed-angle glaucoma
- epilepsy
- disturbance of a warm rhythm
- pregnancy and the period of a lactation
Medicinal interactions
Diazolinum exponentiates effect of the somnolent, sedative and other drugs oppressing the central nervous system and also alcohol.
The special
instructions Diazolinum with care are appointed in a heavy liver and/or renal failure (dose adjustment and increase in intervals between receptions is possible).
During treatment by Diazolinum consumption of alcoholic beverages is not recommended.
Use in pediatrics
Drug in a dosage of 0.05 g is appointed to children is more senior than 3 years.
Drug in a dosage of 0.1 g is appointed to adults and children from 12 years.
The feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
At use of drug is not recommended to run motor transport and to be engaged in other potentially dangerous types of activity which demand concentration of attention.
The overdose
At overdose of drug increases the risk of emergence of by-effects described in appropriate section.
Treatment: drug is cancelled, if necessary hold events of the general detoxication (gastric lavage, an artificial diuresis), symptomatic therapy.
A form of release and packing
On 10 dragees in blister strip packaging from a film of the polyvinylchloride colourless and printing aluminum foil varnished without investment in a pack. In coordination with the consumer the group packing of blister strip packagings in boxes of cardboard together with the corresponding number of instructions for medical use in the state and Russian languages is allowed.
Or on 10 dragees in blister strip packaging from a film of the polyvinylchloride colourless and printing aluminum foil varnished. On the 2nd blister strip packagings together with the instruction for medical use in the state and Russian languages place in a pack from cardboard.
To Store storage conditions in the place protected from light at a temperature not above 25 °C.
To store out of children's reach!
A period of storage
3 years 6 months
not to use drug after the termination of the expiration date specified on packing.
Prescription status
According to the prescription
PJSC Pharmak Producer, Ukraine, 04080, Kiev, st. of Frunze, 63.
The owner of the registration certificate
of PJSC Pharmak, Ukraine
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods) the Republic of Kazakhstan, Almaty, index 050012, Amanagelda St. 59 'A' Business center 'Shartas', the 9th floor. Ph. +7 (727) 367 64 63, fax +7 (727) 267 63 73, e-mail address: Djatlova88@mail.ru
of DIAZOLINUM medicine
the Trade name
Diazolinum
the International unlicensed
name Mebgidrolin Lekarstvennaya a form
of the Dragee of 0.05 and 0.1 g
Structure
One dragee contains
active agent - a mebgidrolin in terms of 100% dry matter of-0.05 g or 0.1 g,
excipients: sucrose, starch syrup, talc, wax yellow, sunflower oil.
Description
of the Dragee of white color. At cross section two layers are visible. The dragee has to have the correct spherical shape. The surface of a dragee has to be equal, smooth and uniform
Pharmacotherapeutic group in color
Antihistamines for system use. Mebgidrolin
ATX R06A X15 Code
the Pharmacological
Pharmacokinetics Quickly properties is soaked up from a digestive tract. The bioavailability fluctuates within 40-60%. The therapeutic effect develops 15-30 minutes later, the maximum action is observed in 1-2 hours. Duration of effect can reach 2 days. Drug practically does not get through a blood-brain barrier, is metabolized in a liver by methylation, induces liver enzymes, is removed from an organism by kidneys.
The pharmacodynamics
Mebgidrolin belongs to antihistaminic drugs, is a blocker of Hl-receptors of a histamine. Mebgidrolin weakens spazmogenny effect of a histamine concerning unstriated muscles of bronchial tubes, intestines and also its influence on permeability of vessels. Unlike antihistaminic drugs of the first generation (Dimedrol, Suprastinum) has less significant sedative and somnolent effect. Possesses slight m-holinoblokiruyushchimi and the anesthetizing properties.
Indications
- prevention and treatment of seasonal and allergic rhinitis
- a pollinosis
- urticaria
- food and medicinal allergy
- skin reactions after stings of insects
- a dermatosis which is followed by a skin itching (eczema, neurodermatitis)
the Route of administration and doses
appoint Diazolinum inside, after a meal, to adults and children from 12 years on 0.1-0.2 g 1-2 times a day. The maximum doses for adults: single – 0.3 g, daily – 0.6 g.
To children of 5-12 years appoint 0.05 g 1-3 times a day, children are 3-5 years old – on 0.05 g 1-2 times a day. Duration of treatment is determined by the doctor depending on the nature of a disease, clinical effect and tolerance of drug.
Side effects
- the irritation of mucous membranes of digestive tract which are sometimes shown by the dispepsichesky phenomena (heartburn, nausea, pains in epigastric area)
- dizziness, paresthesias, increased fatigue, drowsiness, illegibility of visual perception, delay of speed of reactions, a tremor, uneasiness (night)
- dryness in a mouth, urination disturbances, allergic reactions
- is possible emergence of a granulocytopenia and an agranulocytosis
At children paradoxical reactions are sometimes observed: hyperexcitability, tremor, sleep disorders, irritability.
In isolated cases in the post-registration period the following side reactions were noted: headache, itching, rashes, urticaria, Quincke's edema.
Contraindications
- hypersensitivity to drug components
- a peptic ulcer of a stomach and duodenum during aggravation
- inflammatory diseases of digestive tract
- a pylorostenosis
- a prostate hyperplasia
- closed-angle glaucoma
- epilepsy
- disturbance of a warm rhythm
- pregnancy and the period of a lactation
Medicinal interactions
Diazolinum exponentiates effect of the somnolent, sedative and other drugs oppressing the central nervous system and also alcohol.
The special
instructions Diazolinum with care are appointed in a heavy liver and/or renal failure (dose adjustment and increase in intervals between receptions is possible).
During treatment by Diazolinum consumption of alcoholic beverages is not recommended.
Use in pediatrics
Drug in a dosage of 0.05 g is appointed to children is more senior than 3 years.
Drug in a dosage of 0.1 g is appointed to adults and children from 12 years.
The feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
At use of drug is not recommended to run motor transport and to be engaged in other potentially dangerous types of activity which demand concentration of attention.
The overdose
At overdose of drug increases the risk of emergence of by-effects described in appropriate section.
Treatment: drug is cancelled, if necessary hold events of the general detoxication (gastric lavage, an artificial diuresis), symptomatic therapy.
A form of release and packing
On 10 dragees in blister strip packaging from a film of the polyvinylchloride colourless and printing aluminum foil varnished without investment in a pack. In coordination with the consumer the group packing of blister strip packagings in boxes of cardboard together with the corresponding number of instructions for medical use in the state and Russian languages is allowed.
Or on 10 dragees in blister strip packaging from a film of the polyvinylchloride colourless and printing aluminum foil varnished. On the 2nd blister strip packagings together with the instruction for medical use in the state and Russian languages place in a pack from cardboard.
To Store storage conditions in the place protected from light at a temperature not above 25 °C.
To store out of children's reach!
A period of storage
3 years 6 months
not to use drug after the termination of the expiration date specified on packing.
Prescription status
According to the prescription
PJSC Pharmak Producer, Ukraine, 04080, Kiev, st. of Frunze, 63.
The owner of the registration certificate
of PJSC Pharmak, Ukraine
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods) the Republic of Kazakhstan, Almaty, index 050012, Amanagelda St. 59 'A' Business center 'Shartas', the 9th floor. Ph. +7 (727) 367 64 63, fax +7 (727) 267 63 73, e-mail address: Djatlova88@mail.ru