The instruction for medical use
of Dazel Keith medicine
the Trade name Dazel Keith
Mezhdunarodnoye the unlicensed name
Is not present
the Dosage form
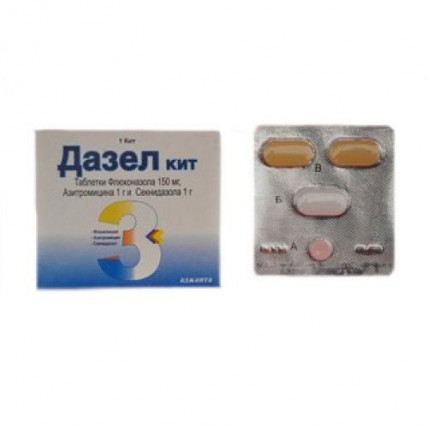
the Combined set:
Flukonazol of a tablet, not coated 150 mg
of Azithromycin of a tablet, coated 1 g
of Seknidazol of a tablet, coated 1 g
Structure
Each whale supports
1) Flukonazola of a tablet of 150 mg (1 tablet)
2) Azithromycin of tablet 1g (1 tablet)
3) Seknidazola of tablet 1g (2 tablets)
Flukonazol
One tablet contains
active agent - flukonazol 150 mg,
excipients: microcrystalline cellulose, corn starch, croscarmellose sodium, colloidal solution of silicon dioxide, magnesium stearate, Ponceau 4R.
Azithromycin
One tablet contains
active agent - azithromycin a dihydrate, equivalent anhydrous azithromycin of 1 g,
excipients: the dimain calcium phosphate, corn starch, sodium a lauryl sulfate, croscarmellose sodium, polyvinyl pirolidon, krospovidon, magnesium stearate, the purified talc, colloidal solution of silicon dioxide, Instacoat white.
Seknidazol
One tablet contains
active agent - seknidazol 1 g,
excipients: corn starch, microcrystalline cellulose, polyvinyl pirolidon, colloidal solution of silicon dioxide, sodium glikolit starch, magnesium stearate, Wincoat yellow.
Flukanazol's description of a tablet: pink, roundish, flat tablets from risky.
Tablet azithromycin: white tablets of the extended form, coated.
Seknidazola of a tablet: yellowish tablets of a capsuloobrazny form, coated, with risky on one party.
Pharmacotherapeutic group
of Antiseptics and antimicrobial drugs for treatment of gynecologic diseases.
Code of automatic telephone exchange G01A
Pharmacological
Flukonazol Pharmacokinetics properties: the bioavailability of drug at intake is 90% that is comparable with indicators at intravenous administration of drug. The maximum concentration in blood plasma is reached within 1-2 hours after intake. Elimination half-life (T1/2) makes about 30 hours. Flukonazol is brought generally with urine, about 80% of the accepted dose are removed in not changed look, about 11% - in the form of metabolites.
Azithromycin: at intake, drug is quickly soaked up in the digestive tract (DT) and widely spreads on an organism. The indicator of linking with proteins of blood plasma depends on concentration drug in blood plasma and makes 51% at concentration 0.02mkg/ml and 7% at concentration 2mkg/ml. Main way of elimination of drug biliary. With bile drug is removed in not changed look. After intake of Azithromycin within a week, about 6% of the accepted dose begin to be removed with urine in not changed look.
Seknidazol: it is slowly and completely absorbed after the oral use, the average term of its action is from about 17 to 29 hours, at the patients suffering from an amebiasis and a giardiasis, response to treatment is observed at a dose 2gr in one day, the patients suffering from a hepatic amebiasis need treatment from 5 to 7 days.
He joins only in 15% plasma proteins.
He well extends on all organism, and did not find high concentration in placentary fabrics. Approximate time of its distribution is 10 minutes. Because of slow absorption, he can remain in intestines more long time and fights in situ in intestines against such parasites as: zhiardiya and amoeba.
The pharmacodynamics
Azithromycin an antibiotic of group of macroleads, has antibacterial effect on Neisseria gonorrhea and Chlamydia trachomatis. Seknidazol is antiprotozoan means, has antiprotozoan and antimicrobial effect. It is effective concerning Trichomonas vaginalis and microorganisms causing bacterial vaginita (Gardnerella vaginalis and anaerobe bacterias).
Flukonazol antifungal drug which is effective concerning Candida albicans.
Indications
- the vaginita caused by microorganisms, sensitive to drug,
the Route of administration and doses
Azithromycin: 1 tablet in 1-2 hours prior to meal.
Flukonazol: 1 tablet to or after meal.
Seknidazol: 2 tablets at meal time.
This complex is intended for single dose in one day of all components. Need of repeated treatment is defined by the attending physician individually.
Azithromycin has to be accepted on a hungry stomach, in 1 hour prior to meal. 2 pill Seknidazola are taken at the same time in the form of a single dose during meal to avoid development of side effects from digestive tract. Flukonazol it is possible to accept along with Seknidazol or separately from him.
Side effects
- a headache, dizziness, an ataxy, paresthesias, a polyneuropathy
- nausea, vomiting, abdominal pains, unpleasant smack in a mouth, diarrhea, a meteorism
- heavy manifestations of hepatotoxicity at patients with liver diseases, cholestatic jaundice
- skin rashes, toxic epidermal necrosis, Stephens-Johnson's syndrome
- a leukopenia,
Contraindication thrombocytopenia
- hypersensitivity to azithromycin, erythromycin or any antibiotic of group of macroleads
- hypersensitivity to a flukonazol or any other component of drug
- hypersensitivity to nitroimidazole derivatives
- pregnancy and the period of a lactation
- organic diseases of the central nervous system
- pathological changes of a picture of peripheral blood (including in the anamnesis)
Medicinal interactions
Flukonazol: at simultaneous use with coumarinic anticoagulants the increase in a prothrombin time is noted. Combined use of a flukanozol and oral hypoglycemic means of patients with diabetes is allowed, however the doctor has to mean a possibility of development of a hypoglycemia. At simultaneous use with rifampicin the shortening of elimination half-life of a flukonazol is noted.
Azithromycin: at simultaneous use with drugs of an ergot the development of an ergotism is possible. At simultaneous use with cyclosporine azithromycin interferes with metabolism of the last therefore risk of development of the side and toxic reactions caused by cyclosporine increases.
Seknidazol: at simultaneous use with anticoagulants the action of the last can amplify. At simultaneous use of a seknidazol with Disulfiramum the development paranoiac reaction and psychosis is possible, with ethanol the development of the effects similar to effect of Disulfiramum is possible. It is not recommended to combine with not depolarizing muscle relaxants (a vekuroniya bromide). At simultaneous use with lithium drugs seknidazol increases its concentration in blood plasma. At a combination to amoxicillin the activity concerning Helicobacter pylori increases.
Special instructions
Flukonazol: it is necessary to appoint drug with care at disturbance of functions of a liver and kidneys. The dose of drug has to be reduced for patients with impaired renal function. In a slight and medium-weight renal failure & frac12, a usual dosage. In a heavy renal failure 1/3 usual dosages.
Azithromycin: it is necessary to appoint with care to patients with dysfunctions baking and cardiac arrhythmias. There are no data on Azithromycin use by patients with impaired renal function and therefore such patients should appoint drug with care.
Seknidazol: during treatment it is necessary to avoid alcohol use
Use in pediatrics
of the Tablet Dazel Keith in pediatric practice are not applied.
The feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
needs to observe extra care during the occupations potentially dangerous types of activity requiring special attention and speed of psychomotor reactions.
Overdose
Symptoms: strengthening of side effects.
Treatment: symptomatic.
A form of release and packing
1 blister containing 4 tablets together with the instruction for use in a cardboard box.
To Store storage conditions in the dry, protected from light place, at a temperature 15C up to 30C.
To store out of children's reach!
2 years
not to apply a period of storage after an expiration date.
Prescription status
According to the prescription
Pharm Limited Adzhanta House Ajanta Producer, 98,
Kandivli Kooperativ Estate Limited,
Charkop, Kandivli (B), Mumbai 400067,
India
to Develop
of Dazel Keith medicine
the Trade name Dazel Keith
Mezhdunarodnoye the unlicensed name
Is not present
the Dosage form
the Combined set:
Flukonazol of a tablet, not coated 150 mg
of Azithromycin of a tablet, coated 1 g
of Seknidazol of a tablet, coated 1 g
Structure
Each whale supports
1) Flukonazola of a tablet of 150 mg (1 tablet)
2) Azithromycin of tablet 1g (1 tablet)
3) Seknidazola of tablet 1g (2 tablets)
Flukonazol
One tablet contains
active agent - flukonazol 150 mg,
excipients: microcrystalline cellulose, corn starch, croscarmellose sodium, colloidal solution of silicon dioxide, magnesium stearate, Ponceau 4R.
Azithromycin
One tablet contains
active agent - azithromycin a dihydrate, equivalent anhydrous azithromycin of 1 g,
excipients: the dimain calcium phosphate, corn starch, sodium a lauryl sulfate, croscarmellose sodium, polyvinyl pirolidon, krospovidon, magnesium stearate, the purified talc, colloidal solution of silicon dioxide, Instacoat white.
Seknidazol
One tablet contains
active agent - seknidazol 1 g,
excipients: corn starch, microcrystalline cellulose, polyvinyl pirolidon, colloidal solution of silicon dioxide, sodium glikolit starch, magnesium stearate, Wincoat yellow.
Flukanazol's description of a tablet: pink, roundish, flat tablets from risky.
Tablet azithromycin: white tablets of the extended form, coated.
Seknidazola of a tablet: yellowish tablets of a capsuloobrazny form, coated, with risky on one party.
Pharmacotherapeutic group
of Antiseptics and antimicrobial drugs for treatment of gynecologic diseases.
Code of automatic telephone exchange G01A
Pharmacological
Flukonazol Pharmacokinetics properties: the bioavailability of drug at intake is 90% that is comparable with indicators at intravenous administration of drug. The maximum concentration in blood plasma is reached within 1-2 hours after intake. Elimination half-life (T1/2) makes about 30 hours. Flukonazol is brought generally with urine, about 80% of the accepted dose are removed in not changed look, about 11% - in the form of metabolites.
Azithromycin: at intake, drug is quickly soaked up in the digestive tract (DT) and widely spreads on an organism. The indicator of linking with proteins of blood plasma depends on concentration drug in blood plasma and makes 51% at concentration 0.02mkg/ml and 7% at concentration 2mkg/ml. Main way of elimination of drug biliary. With bile drug is removed in not changed look. After intake of Azithromycin within a week, about 6% of the accepted dose begin to be removed with urine in not changed look.
Seknidazol: it is slowly and completely absorbed after the oral use, the average term of its action is from about 17 to 29 hours, at the patients suffering from an amebiasis and a giardiasis, response to treatment is observed at a dose 2gr in one day, the patients suffering from a hepatic amebiasis need treatment from 5 to 7 days.
He joins only in 15% plasma proteins.
He well extends on all organism, and did not find high concentration in placentary fabrics. Approximate time of its distribution is 10 minutes. Because of slow absorption, he can remain in intestines more long time and fights in situ in intestines against such parasites as: zhiardiya and amoeba.
The pharmacodynamics
Azithromycin an antibiotic of group of macroleads, has antibacterial effect on Neisseria gonorrhea and Chlamydia trachomatis. Seknidazol is antiprotozoan means, has antiprotozoan and antimicrobial effect. It is effective concerning Trichomonas vaginalis and microorganisms causing bacterial vaginita (Gardnerella vaginalis and anaerobe bacterias).
Flukonazol antifungal drug which is effective concerning Candida albicans.
Indications
- the vaginita caused by microorganisms, sensitive to drug,
the Route of administration and doses
Azithromycin: 1 tablet in 1-2 hours prior to meal.
Flukonazol: 1 tablet to or after meal.
Seknidazol: 2 tablets at meal time.
This complex is intended for single dose in one day of all components. Need of repeated treatment is defined by the attending physician individually.
Azithromycin has to be accepted on a hungry stomach, in 1 hour prior to meal. 2 pill Seknidazola are taken at the same time in the form of a single dose during meal to avoid development of side effects from digestive tract. Flukonazol it is possible to accept along with Seknidazol or separately from him.
Side effects
- a headache, dizziness, an ataxy, paresthesias, a polyneuropathy
- nausea, vomiting, abdominal pains, unpleasant smack in a mouth, diarrhea, a meteorism
- heavy manifestations of hepatotoxicity at patients with liver diseases, cholestatic jaundice
- skin rashes, toxic epidermal necrosis, Stephens-Johnson's syndrome
- a leukopenia,
Contraindication thrombocytopenia
- hypersensitivity to azithromycin, erythromycin or any antibiotic of group of macroleads
- hypersensitivity to a flukonazol or any other component of drug
- hypersensitivity to nitroimidazole derivatives
- pregnancy and the period of a lactation
- organic diseases of the central nervous system
- pathological changes of a picture of peripheral blood (including in the anamnesis)
Medicinal interactions
Flukonazol: at simultaneous use with coumarinic anticoagulants the increase in a prothrombin time is noted. Combined use of a flukanozol and oral hypoglycemic means of patients with diabetes is allowed, however the doctor has to mean a possibility of development of a hypoglycemia. At simultaneous use with rifampicin the shortening of elimination half-life of a flukonazol is noted.
Azithromycin: at simultaneous use with drugs of an ergot the development of an ergotism is possible. At simultaneous use with cyclosporine azithromycin interferes with metabolism of the last therefore risk of development of the side and toxic reactions caused by cyclosporine increases.
Seknidazol: at simultaneous use with anticoagulants the action of the last can amplify. At simultaneous use of a seknidazol with Disulfiramum the development paranoiac reaction and psychosis is possible, with ethanol the development of the effects similar to effect of Disulfiramum is possible. It is not recommended to combine with not depolarizing muscle relaxants (a vekuroniya bromide). At simultaneous use with lithium drugs seknidazol increases its concentration in blood plasma. At a combination to amoxicillin the activity concerning Helicobacter pylori increases.
Special instructions
Flukonazol: it is necessary to appoint drug with care at disturbance of functions of a liver and kidneys. The dose of drug has to be reduced for patients with impaired renal function. In a slight and medium-weight renal failure & frac12, a usual dosage. In a heavy renal failure 1/3 usual dosages.
Azithromycin: it is necessary to appoint with care to patients with dysfunctions baking and cardiac arrhythmias. There are no data on Azithromycin use by patients with impaired renal function and therefore such patients should appoint drug with care.
Seknidazol: during treatment it is necessary to avoid alcohol use
Use in pediatrics
of the Tablet Dazel Keith in pediatric practice are not applied.
The feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
needs to observe extra care during the occupations potentially dangerous types of activity requiring special attention and speed of psychomotor reactions.
Overdose
Symptoms: strengthening of side effects.
Treatment: symptomatic.
A form of release and packing
1 blister containing 4 tablets together with the instruction for use in a cardboard box.
To Store storage conditions in the dry, protected from light place, at a temperature 15C up to 30C.
To store out of children's reach!
2 years
not to apply a period of storage after an expiration date.
Prescription status
According to the prescription
Pharm Limited Adzhanta House Ajanta Producer, 98,
Kandivli Kooperativ Estate Limited,
Charkop, Kandivli (B), Mumbai 400067,
India
to Develop