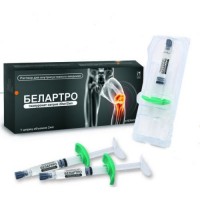
Chondro Gialurom 60 mg / 3 ml and 90 mg / 3 ml 1's solution for intraarticular administration in the syringe
- $238.30
Sku:
246bb95effeb
Brand:
Rompharm (Romania)
The instruction
for medical use of a product of medical purpose for the consumer
the Name of a product of medical purpose
by Gialur Hondro, solution for intra articulate introduction of 60 mg / 3 ml and 90 mg / 3 ml, the prefilled syringe of 3 ml,
the No. 1 Structure and the description
of the product The Prefilled Syringe with solution for intra articulate introduction of 3 ml, No. 1.
3 ml of solution contain sodium hyaluronate of 60 mg, chondroitin sodium sulfate of 90 mg, sodium chloride of 10.50 mg, sodium dihydrophosphate monohydrate of 1.35 mg, sodium hydrophosphate dodecahydrate of 15.15 mg, 1 M solution of sodium of hydroxide or 1 M acid chlorohydrogen to pH = 7.4 & plusmn, 0.2 and water for injections to 3 ml.
Gialur Hondro is transparent, from colourless till brown color viscous solution.
Gialur Hondro is issued in the form of the prefilled syringe from transparent glass of the first hydrolytic class siliconized, equipped with an elastomeric cap and an emphasis for fingers which contains not less than 3 ml of sterile solution.
On each prefilled syringe paste the label.
On 1 prefilled syringe place in strip plastic packaging with a covering from the marked aluminum foil or an unmarked polyethylene transparent film. On 1 strip plastic packaging with a disposable sterile needle 21 G x 1 1/2 together with the instruction for medical use in the state and Russian languages in a folding cardboard box.
A scope
For treatment of diseases of the musculoskeletal system:
Works with Gialur Hondro as temporary replacement and addition of synovial fluid.
By Gialur Hondro it is intended for symptomatic treatment of an osteoarthritis, from a lung to heavy.
Gialur Hondro treats the pain and reduced mobility caused by degenerative or traumatic pathologies of a knee and other synovial joints (hip, an anklebone, a shoulder, an elbow, a hand wrist, fingers, a temporal and mandibular joint, dugootroschaty joints).
Gialur Hondro kills a joint pain, improves mobility of joints and protects a cartilage.
By Gialur Hondro it is also shown for pain relief after an arthroscopy.
By Gialur Hondro it is intended for recovery of viscosity of synovial fluid. This therapy is safe, effective and well reasonable at treatment of an osteoarthritis and consists in administration of solution on the basis of hyaluronic acid in an affected synovial joint.
The Hyaluronic Acid (HA) is the main component of synovial fluid and a cartilage and thanks to the viscoelastic and rheological properties is responsible for lubricant and depreciation in joints. It reduces friction between articulate surfaces and protects soft tissues from injuries, working as the shock shock-absorber. Hyaluronic Acid also has anesthetic, anti-inflammatory, antioxidant, hondroprotektorny action and stimulates synthesis of proteoglycans.
The quantity and quality of group of companies in synovial fluid decreases at patients with an osteoarthritis as its synthesis by synovial cells and cells of a cartilage is broken. Thus, protection of surfaces of a joint strongly changes, the cartilage becomes vulnerable and is exposed to structural damages because of friction forces and compression.
Gialur Hondro, entered as one injection, will restore good lubricant and depreciation in joints it is considerable also for long term, will relieve pain and rigidity of joints.
A route of administration
the Drug is administered by an intra articulate injection.
Aseptic technology of introduction has to be observed strictly.
The place of an injection has to be properly disinfected (alcohol of 70% or other disinfectants). The disinfectants containing salts of quarternary ammonium as hyaluronic acid can be besieged in these conditions should not be used.
Before introduction by Gialur Hondro, any accumulation of liquid in joints has to be removed by exhaustion with the syringe.
The ready syringe is removed from sterile strip plastic packaging, the cap is removed from a syringe tip then the sterile needle which is fixed by small turn is put on. Before introduction it is necessary to remove air from the syringe.
The amount of the administered drug Gialurom Hondro depends on the joint size. The intra articulate space should not be overloaded.
Each prefilled syringe is intended for single use. Contents of the prefilled syringe are sterile and have to be used immediately after packing opening. Throw out unused Gialur Hondro. Drug should not be repeatedly sterilized.
Gialur Hondro it is entered only by the doctors trained technology of intra articulate introduction.
Hypodermic administration of lidocaine or similar anesthetic can be recommended before Gialurom Hondro's introduction.
Completeness
No.
Components
the manufacturing Organization (manufacturer)
the Country
1
Prefilled syringe from 3 ml of solution for intra articulate introduction, No. 1.
3 ml of solution contain sodium hyaluronate of 60 mg, chondroitin sodium sulfate of 90 mg, sodium chloride of 10.50 mg, sodium digidro-phosphate monohydrate of 1.35 mg, sodium hydrophosphate dodecahydrate of 15.15 mg, 1 M solution of sodium of hydroxide or 1 M acid chlorohydrogen to pH = 7.4 & plusmn, 0.2 and water for injections to 3 ml.
The syringe with a capacity of 3 ml consists from:
The syringe body from the colourless
Piston Rod Emphasis glass for fingers
BD Medical Pharmaceutical Systems
France
2
Syringe needle of 21
G x 1 1/2 CHIRANA T. Injecta, a.s.
Slovakia
Storage conditions
At a temperature not higher than 25 of 0C, in original packing.
Not to freeze.
To store out of children's reach!
2 years
not to apply an expiration date after expiry date.
It is necessary to check expiry date of the validity and integrity of the package before use.
Not to use after the expiry date specified on packing or if packing is damaged.
The used needles and syringes have to be destroyed after each injection and should not be used for other injection.
The Rompharm Company manufacturing organization
of S.R. L. name of the normative document
the Specification on a product of medical purpose by Gialur Hondro, solution for intra articulate introduction of 60 mg / 3 ml and 90 mg / 3 ml, the prefilled syringe of 3 ml, No. 1
Side effect
Hypostasis and the passing pain can arise after an injection. These reactions usually disappear within 72 hours.
Contraindications for use
& middot, hypersensitivity (including hypersensitivity in the anamnesis) to the Gialurom Hondro components
& middot, patients with infections or diseases of skin in the place of an injection
& middot, children and teenagers
& middot, pregnant women and the feeding women
& middot, the known system disturbances of blood clotting
of the Precautionary measure (safety)
it is vnesustavno not necessary to administer the Drug.
Injections Gialur Hondro have to be made by exclusively diplomaed and certified doctors of the corresponding profile.
It is not necessary to use Gialur Hondro at the same time or in mix with other drugs intended for intra articulate use.
The drug is administered only if solution is transparent.
Measures of first-aid treatment at misuse or collateral influence.
Similar to other invasive procedures in joints, the patient is recommended to adhere to the sparing mode and to avoid excessive load of a joint within 48 hours after an intra articulate injection Gialur Hondro.
Patients with painful consequences after intra articulate introduction by Gialur Hondro should see a doctor immediately. If pain amplifies during introduction, the procedure has to be stopped.
for medical use of a product of medical purpose for the consumer
the Name of a product of medical purpose
by Gialur Hondro, solution for intra articulate introduction of 60 mg / 3 ml and 90 mg / 3 ml, the prefilled syringe of 3 ml,
the No. 1 Structure and the description
of the product The Prefilled Syringe with solution for intra articulate introduction of 3 ml, No. 1.
3 ml of solution contain sodium hyaluronate of 60 mg, chondroitin sodium sulfate of 90 mg, sodium chloride of 10.50 mg, sodium dihydrophosphate monohydrate of 1.35 mg, sodium hydrophosphate dodecahydrate of 15.15 mg, 1 M solution of sodium of hydroxide or 1 M acid chlorohydrogen to pH = 7.4 & plusmn, 0.2 and water for injections to 3 ml.
Gialur Hondro is transparent, from colourless till brown color viscous solution.
Gialur Hondro is issued in the form of the prefilled syringe from transparent glass of the first hydrolytic class siliconized, equipped with an elastomeric cap and an emphasis for fingers which contains not less than 3 ml of sterile solution.
On each prefilled syringe paste the label.
On 1 prefilled syringe place in strip plastic packaging with a covering from the marked aluminum foil or an unmarked polyethylene transparent film. On 1 strip plastic packaging with a disposable sterile needle 21 G x 1 1/2 together with the instruction for medical use in the state and Russian languages in a folding cardboard box.
A scope
For treatment of diseases of the musculoskeletal system:
Works with Gialur Hondro as temporary replacement and addition of synovial fluid.
By Gialur Hondro it is intended for symptomatic treatment of an osteoarthritis, from a lung to heavy.
Gialur Hondro treats the pain and reduced mobility caused by degenerative or traumatic pathologies of a knee and other synovial joints (hip, an anklebone, a shoulder, an elbow, a hand wrist, fingers, a temporal and mandibular joint, dugootroschaty joints).
Gialur Hondro kills a joint pain, improves mobility of joints and protects a cartilage.
By Gialur Hondro it is also shown for pain relief after an arthroscopy.
By Gialur Hondro it is intended for recovery of viscosity of synovial fluid. This therapy is safe, effective and well reasonable at treatment of an osteoarthritis and consists in administration of solution on the basis of hyaluronic acid in an affected synovial joint.
The Hyaluronic Acid (HA) is the main component of synovial fluid and a cartilage and thanks to the viscoelastic and rheological properties is responsible for lubricant and depreciation in joints. It reduces friction between articulate surfaces and protects soft tissues from injuries, working as the shock shock-absorber. Hyaluronic Acid also has anesthetic, anti-inflammatory, antioxidant, hondroprotektorny action and stimulates synthesis of proteoglycans.
The quantity and quality of group of companies in synovial fluid decreases at patients with an osteoarthritis as its synthesis by synovial cells and cells of a cartilage is broken. Thus, protection of surfaces of a joint strongly changes, the cartilage becomes vulnerable and is exposed to structural damages because of friction forces and compression.
Gialur Hondro, entered as one injection, will restore good lubricant and depreciation in joints it is considerable also for long term, will relieve pain and rigidity of joints.
A route of administration
the Drug is administered by an intra articulate injection.
Aseptic technology of introduction has to be observed strictly.
The place of an injection has to be properly disinfected (alcohol of 70% or other disinfectants). The disinfectants containing salts of quarternary ammonium as hyaluronic acid can be besieged in these conditions should not be used.
Before introduction by Gialur Hondro, any accumulation of liquid in joints has to be removed by exhaustion with the syringe.
The ready syringe is removed from sterile strip plastic packaging, the cap is removed from a syringe tip then the sterile needle which is fixed by small turn is put on. Before introduction it is necessary to remove air from the syringe.
The amount of the administered drug Gialurom Hondro depends on the joint size. The intra articulate space should not be overloaded.
Each prefilled syringe is intended for single use. Contents of the prefilled syringe are sterile and have to be used immediately after packing opening. Throw out unused Gialur Hondro. Drug should not be repeatedly sterilized.
Gialur Hondro it is entered only by the doctors trained technology of intra articulate introduction.
Hypodermic administration of lidocaine or similar anesthetic can be recommended before Gialurom Hondro's introduction.
Completeness
No.
Components
the manufacturing Organization (manufacturer)
the Country
1
Prefilled syringe from 3 ml of solution for intra articulate introduction, No. 1.
3 ml of solution contain sodium hyaluronate of 60 mg, chondroitin sodium sulfate of 90 mg, sodium chloride of 10.50 mg, sodium digidro-phosphate monohydrate of 1.35 mg, sodium hydrophosphate dodecahydrate of 15.15 mg, 1 M solution of sodium of hydroxide or 1 M acid chlorohydrogen to pH = 7.4 & plusmn, 0.2 and water for injections to 3 ml.
The syringe with a capacity of 3 ml consists from:
The syringe body from the colourless
Piston Rod Emphasis glass for fingers
BD Medical Pharmaceutical Systems
France
2
Syringe needle of 21
G x 1 1/2 CHIRANA T. Injecta, a.s.
Slovakia
Storage conditions
At a temperature not higher than 25 of 0C, in original packing.
Not to freeze.
To store out of children's reach!
2 years
not to apply an expiration date after expiry date.
It is necessary to check expiry date of the validity and integrity of the package before use.
Not to use after the expiry date specified on packing or if packing is damaged.
The used needles and syringes have to be destroyed after each injection and should not be used for other injection.
The Rompharm Company manufacturing organization
of S.R. L. name of the normative document
the Specification on a product of medical purpose by Gialur Hondro, solution for intra articulate introduction of 60 mg / 3 ml and 90 mg / 3 ml, the prefilled syringe of 3 ml, No. 1
Side effect
Hypostasis and the passing pain can arise after an injection. These reactions usually disappear within 72 hours.
Contraindications for use
& middot, hypersensitivity (including hypersensitivity in the anamnesis) to the Gialurom Hondro components
& middot, patients with infections or diseases of skin in the place of an injection
& middot, children and teenagers
& middot, pregnant women and the feeding women
& middot, the known system disturbances of blood clotting
of the Precautionary measure (safety)
it is vnesustavno not necessary to administer the Drug.
Injections Gialur Hondro have to be made by exclusively diplomaed and certified doctors of the corresponding profile.
It is not necessary to use Gialur Hondro at the same time or in mix with other drugs intended for intra articulate use.
The drug is administered only if solution is transparent.
Measures of first-aid treatment at misuse or collateral influence.
Similar to other invasive procedures in joints, the patient is recommended to adhere to the sparing mode and to avoid excessive load of a joint within 48 hours after an intra articulate injection Gialur Hondro.
Patients with painful consequences after intra articulate introduction by Gialur Hondro should see a doctor immediately. If pain amplifies during introduction, the procedure has to be stopped.