-10%
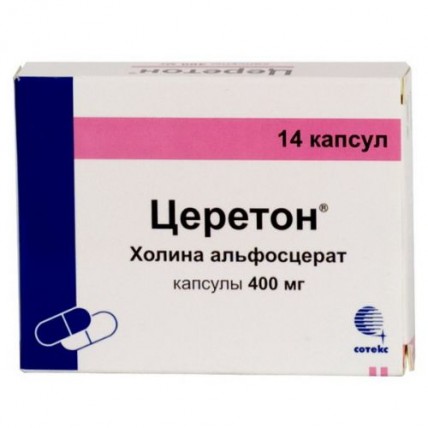
Cereton® (alpha-GPC) 400 mg (14 capsules)
- $38.00
- $42.00
STRUCTURE
ONE CAPSULE CONTAINS
THE ACTIVE SUBSTANCE - CHOLINE ALFOSCERATE IN CONVERSION OF 100% SUBSTANCE - 400 MG,
AUXILIARY SUBSTANCES: GLYCERIN, PURIFIED WATER
PHARMACOLOGICAL PROPERTIES
PHARMACOKINETICS
SUCTION AND DISTRIBUTION
AFTER TAKING INSIDE, ABSORPTION IS 88%. EASILY PENETRES THROUGH THE HEMATOENCEPHALIC BARRIER, ACCUMULATES PREVIOUSLY IN THE BRAIN (CONCENTRATION REACHES 45% OF THE LEVEL IN BLOOD PLASMA), LUNGS AND LIVER.
METABOLISM AND WITHDRAWAL
85% IS EXCLUDED BY LUNG IN THE FORM OF CARBON DIOXIDE, THE REST (15%) - BY THE KIDNEYS AND THROUGH THE INTESTINAL.
PHARMACODYNAMICS
Choline alphosceratus IS A transport form of choline and the precursor of phosphatidylcholine, which is potentially capable of preventing damage to the membrane, as a factor in pathogenesis psychoorganic involutional syndrome, characterized by a decrease of cholinergic synaptic transmission and damaged neuronal membrane phospholipid composition. THE CHEMICAL STRUCTURE OF CHOLINE ALPHOSCERATE (CONTAINING 40.5% CHOLINE) AND THE PHYSICAL AND CHEMICAL PROPERTIES OF THE MOLECULE ASSOCIATED WITH IT PROVIDE PENETRATION OF A PART OF CHOLINE THROUGH THE HEMATOENCERIC BALANCE. THE RESULTS OF PRE-CLINICAL AND CLINICAL STUDIES CONFIRM THE ABILITY OF CHOLINE ALFOSCERATE TO INFLUENCE PREVENTIVELY ON COGNITIVE-MESTIC FUNCTIONS, AS WELL AS ON EMOTIONAL AND EFFECTIVE MOSCULENCE.
INDICATIONS FOR USE
- degenerative and involutional psychoorganic syndrome and consequences of cerebrovascular insufficiency, such as primary and secondary disturbances mnemonic function characterizing memory impairment, confusion, disorientation, lack of motivation, initiative and the ability to concentrate;
- CHANGES IN THE EMOTIONAL AND BEHAVIORAL SPHERE OF THE ELDERLY: EMOTIONAL LABILITY, INCREASED IRRITANCY, REDUCED INTEREST IN THE ENVIRONMENT, SENIER PSEUDO DEPRESSION
METHOD OF APPLICATION AND DOSE
CAPSULES TAKE INSIDE 400 MG (1 CAPSULE) 2-3 TIMES PER DAY.
DAILY DOSE MAY BE INCREASED AT THE DISCIPLINE OF THE DOCTOR. THE DURATION OF THE COURSE OF TREATMENT IS DETERMINED BY THE DOCTOR, taking into account the DISEASE, TOLERANCE OF THE DRUG AND THE EFFECT ACHIEVED.
SIDE EFFECTS
- Nausea (AS A CONSEQUENCE OF DOPAMINERGIC ACTIVATION);
- ALLERGIC REACTIONS
VERY RARE: ABDOMINAL PAIN, SHORT-TERM CONSCIOUSNESS.
IN THE CASE OF ADVERSE REACTIONS, INCLUDING THOSE NOT SPECIFIED IN THE INSTRUCTIONS FOR MEDICAL APPLICATION, IT IS NECESSARY TO CONSULT A DOCTOR.
CONTRAINDICATIONS
- INCREASED SENSITIVITY TO THE COMPONENTS OF THE PREPARATION;
- PREGNANCY AND LACTATION PERIOD;
- CHILDREN AND ADOLESCENTS UP TO 18 YEARS
DRUG INTERACTIONS
NO CLINICALLY SIGNIFICANT INTERACTION WITH OTHER DRUGS HAS BEEN DETECTED.
SPECIAL INSTRUCTIONS
USE IN CHILDREN
EFFICIENCY AND SAFETY OF USE OF THE PREPARATION IN CHILDREN IS NOT STUDYED ENOUGH. THE MEDICINE IS NOT INTENDED FOR USE IN CHILDREN.
The composition of the shell of the capsules includes parabens, which can cause allergic reactions (possibly of a delayed type).
PECULIARITIES OF THE EFFECT OF A DRUG ON THE ABILITY TO DRIVE A VEHICLE OR POTENTIALLY DANGEROUS MECHANISMS
CARE SHOULD BE TAKEN WHEN DRIVING VEHICLES OR OTHER ACTIVITIES REQUIRING AN INCREASED CONCENTRATION OF ATTENTION AND / OR RAPID MENTAL REACTIONS.
OVERDOSE
SYMPTOMS: Nausea.
TREATMENT: AT THE APPEARANCE OF THIS SYMPTOM IT IS RECOMMENDED TO REDUCE THE DOSE
STORAGE CONDITIONS
STORE IN A DRY, PROTECTED FROM LIGHT AT A TEMPERATURE NOT EXCEEDING 25 ° C.
KEEP OUT OF THE REACH OF CHILDREN!
SHELF LIFE - 3 YEARS
DO NOT USE AFTER THE SHELF LIFE STATED ON THE PACKAGE.