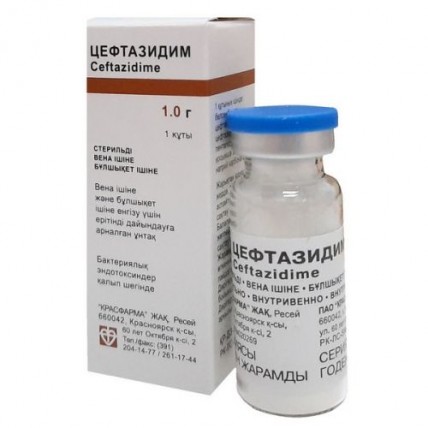
Ceftazidime 1g 1's powder for solution intravenously and intramuscularly
- $7.00
The instruction for medical use of CEFTAZIDIME medicine the Trade name the Ceftazidime the International unlicensed name Ceftazidime Dosage Form Powder for preparation of solution for intravenous and intramuscular administration of 0.5 g, 1.0 g Structure 1 bottle contains active agent: a ceftazidime pentahydrate in terms of a ceftazidime - 0.5 g, 1.0 g excipient: sodium a carbonate the Description Powder from color, white to white with a yellowish shade. Pharmacotherapeutic group Antibacterial drugs for system use. Beta laktamnye antibacterial drugs other. Cephalosporins of the third generation. Ceftazidime. ATX J01DD02 code Pharmacological Pharmacokinetics properties. The maximum concentration (Cmax) after intramuscular introduction (in oil) in doses of 0.5 g and 1.0 g about 17 mg/l and 39 mg/l respectively, time of achievement of the maximum concentration (TCmax) about 1 h Cmax after intravenous (in / c) bolyusny introduction in doses of 0.5 g, 1 g and 2 g about 42 mg/l, 69 mg/l and 170 mg/l respectively. Therapeutic effective serumal concentration remain in 8-12 h after intravenously and intramusculary introductions. Communication with proteins of plasma - less than 10%. The concentration of a ceftazidime exceeding the minimum overwhelming concentration for the majority of widespread pathogenic microorganisms can be reached in a bone tissue, tissues of heart, bile, a phlegm, synovial fluid, intraocular, pleural and peritoneal liquids. Easily gets through a placenta and it is allocated with breast milk. In the absence of inflammatory process badly gets through a blood-brain barrier. In meningitis the concentration in cerebrospinal fluid reaches therapeutic value (4-20 mg/l and above). Elimination half-life (T1/2) - 1.9 h, at newborns - is 3-4 times more long, at a hemodialysis - 3-5 h. It is not metabolized in a liver. It is removed by kidneys (80-90% in not changed look by glomerular filtration) during 24 h, with bile - less than 1%. A pharmacodynamics the Tsefalosporinovy antibiotic of the III generation for parenteral use. Works bakteritsidno (breaks synthesis of a cell wall of microorganisms). Possesses a broad spectrum of activity. It is resistant to action of the majority beta laktamaz. Affects many strains resistant to ampicillin and other cephalosporins. It is active concerning gram-negative microorganisms: Pseudomonas spp., including Pseudomonas aeruginosa, Klebsiella spp., including Klebsiella pneumoniae, Proteus mirabilis, Proteus vulgaris, Escherichia coli, Enterobacter spp., including Enterobacter aerogenes, Enterobacter cloacae, Citrobacter spp., including Citrobacter diversus, Citrobacter freundii, Pasteurella multocida, Neisseria meningitidis, Haemophilus influenzae (including the strains resistant to ampicillin), gram-positive microorganisms: Staphylococcus aureus (which are producing and not producing penicillinase the strains sensitive to Methicillinum), Streptococcus pyogenes (group A a beta and hemolytic streptococcus), Streptococcus agalactiae (group B), Streptococcus pneumoniae, anaerobic microorganisms: Bacteroides spp. (many strains of Bacteroides fragilis of a rezistentna). It is inactive concerning resistant to Methicillinum Staphylococcus spp., Streptococcus faecalis, Enterococcus spp., Listeria monocytogenes, Campylobacter spp. and Clostridium difficile. in vitro against the majority of strains of the following organisms is active: Clostridium perfringens, not including Clostridium difficile, Acinetobacter spp., Haemophilus parainfluenzae, Morganella morganii, Neisseria gonorrhoeae, Peptococcus spp., Peptostreptococcus spp., Providencia spp., Providencia rettgeri, Salmonella spp., Shigella spp., Staphylococcus epidermidis, Yersinia enterocolitica. Indications the Infectious and inflammatory diseases caused by activators, sensitive to a ceftazidime: - the lower airways (bronchitis, the infected bronchiectasias, pneumonia, lung abscess, an empyema of a pleura, an infection of lungs at patients with a mucoviscidosis) - ENT organs (average otitis, sinusitis) - urinary tract (pyelonephritis, a pyelitis, cystitis, an urethritis, abscess of a kidney, infections associated with an urolithiasis) - soft tissues (phlegmon, an ugly face, wound fevers, mastitis, a skin ulcer) - bones and joints (osteomyelitis, septic arthritis) - digestive tract - biliary tract and an abdominal cavity (cholangitis, cholecystitis, an empyema of a gall bladder, retroperitoneal abscesses, peritonitis, a diverticulitis, a coloenteritis) - bodies of a small pelvis - prostatitis - gonorrhea - sepsis - meningitis Prevention of infectious complications at operations on a prostate. A route of administration and doses Intravenously (in / c) or intramusculary (in oil). The dose of drug is established individually, taking into account weight of a course of the disease, localization of an infection and sensitivity of the activator, age and body weight, function of kidneys. To adults and children 12 years are more senior each 8-12 h or on 2 g with an interval of 12 h appoint 1 g. At a severe disease, especially at patients with reduced immunity (including patients with a neutropenia) - on 2 g each 8 h or on 3 g each 12 h. In uncomplicated infections of urinary tract - on 0.25 g 2 times a day. At the complicated infections of urinary tract - on 0.5 - 1 g 2 times a day. In a mucoviscidosis, to patients with the infections of a respiratory system caused by Pseudomonas spp. - on 30-50 mg/kg each 8 h. At operations on a prostate in the preventive purposes enter before anesthesia induction - 1 g, repeat introduction after removal of a catheter. To patients of advanced age the maximum daily dose - 3 g. To children 2 months are more senior and up to 12 years appoint 30-100 mg/kg/days (for 2-3 introductions), to children with reduced immunity, a mucoviscidosis and meningitis - 150 mg/kg/days in 3 introductions, the maximum daily dose - 6 g. To newborns and babies aged up to 2 months appoint 25-60 mg/kg/days in 2 introductions. In a renal failure an initial dose - 1 g. The maintenance dose is selected depending on the clearance of creatinine (CC): The Clearance of Creatinine (CC) the Dose of 50-31 ml/min. 1 g 2 times in day of 30-16 ml/min. 1 g of 1 times in day of 15-6 ml/min. 0.5 g of 1 times a day less than 5 ml/min. 0.5 g of 1 times in 48 h the Patients with infections of a heavy course can increase a single dose by 50%, at the same time at them it is necessary to control concentration of a ceftazidime in blood serum (should not exceed 40 mg/l). For children the clearance of creatinine (CC) is calculated according to the ideal mass or surface area of a body. Against the background of a hemodialysis the maintenance doses are calculated taking into account KK, introduction is carried out after each session of a hemodialysis. Against the background of peritoneal dialysis besides in/in introductions the ceftazidime can be included in dialysis solution (125-250 mg on 2 l of dialysis solution). At the patients with a renal failure who are on a continuous hemodialysis with use of the arteriovenous shunt and at the patients who are on haemo filtration of high speed in intensive care unit, the recommended doses - 1 g/days daily (for one or several introductions). At the patients who are on haemo filtration of low speed appoint the doses recommended in a renal failure. Treatment duration a ceftazidime is 7-14 days. In the infections caused by Pseudomonas aeruginosa (pneumonia, infectious complications in a mucoviscidosis, meningitis) the course of treatment can be increased up to 21 days. Preparation of solution for injections. For introduction in oil the contents of a bottle are dissolved in 1.5 ml (0.5 d) also by 3 ml (1.0 d) solvent (water for injections, 0.5-1% hydrochloride lidocaine solution). At treatment of children aged till 1 year it is not necessary to dissolve drug in lidocaine solutions. For in/in bolyusny the introductions dissolve contents of a bottle in 5 ml (0.5 d) and 10 ml (1.0 g) solvent (water for injections). For in/in drop the introductions in addition dissolve the received drug solution in 50 ml of solvent. At the received ready solution there can be small vials of carbon dioxide that does not affect efficiency of drug. It is impossible to use Natrii hydrocarbonas solution as solvent. Pharmaceutical we will combine with the following solutions: At concentration from 1 to 40 mg/ml - 0.9% chloride sodium solution, lactate sodium solution, Hartman's solution, 5% and 10% solutions of a dextrose, 0.225% solution of sodium of chloride and 5% solution of a dextrose, 0.45% solution of sodium of chloride and 5% solution of a dextrose, 0.9% solution of sodium of chloride and 5% solution of a dextrose, 0.18% solution of sodium of chloride and 4% solution of a dextrose, 10% solution of the Dextran 40 in 0.9% solution of sodium of chloride or in 5% solution of a dextrose, 6% solution of the Dextran 70 in 0.9% solution of sodium of chloride or in 5% a dextrose.rastvor. In concentration from 0:05 mg/ml to 0:25 mg/ml the ceftazidime is compatible to solution for intraperitoneal dialysis (lactate). Both components keep activity if the ceftazidime to concentration of 4 mg/ml is added to the following solutions: a hydrocortisone of 1 mg/ml in 0.9% solution of sodium of chloride or 5% solution of a dextrose, tsefuroksy 3 mg/ml in 0.9% chloride sodium solution, kloksatsillin 4 mg/ml in 0.9% chloride sodium solution, ME/ml heparin 10 or 50 ME/ml in 0.9% solution of sodium of chloride, potassium IEC/l chloride 10 or 40 IEC/l in 0.9% chloride sodium solution. At ceftazidime solution mixing (0.5 g in 1.5 ml of water for injections) and metronidazole (0.5 g / 100 ml) both components keep the activity. To use only freshly cooked solution! Easy yellowing of solution does not affect efficiency. Side effects - phlebitis or thrombophlebitis at intravenous administration, pain, burning, consolidation in the place of an injection at intramuscular introduction - spotty and papular rash, a small tortoiseshell, fever, an itching, a Quincke's disease, a bronchospasm, a lowering of arterial pressure, an exudative multiformny erythema (including Stephens-Johnson's syndrome), a toxic epidermal necrolysis (Lyell's disease) - diarrhea, nausea, vomiting, abdominal pains, orofaringealny candidiasis, colitis, pseudomembranous colitis - a mycotic vulvovaginitis, a renal failure - jaundice - a headache, dizziness, paresthesias, disturbance of flavoring feelings, a tremor, a myoclonia, spasms, encephalopathy, a coma - an eosinophilia, false positive forward reaction of Koombs, a thrombocytosis, increase in activity of "hepatic" enzymes - ALT, AST, LDG, GGTP and alkaline phosphatase, increase in level of urea, urea nitrogen and/or creatinine in blood - a leukopenia, a neutropenia, an agranulocytosis, thrombocytopenia, a lymphocytosis, hemolytic anemia - the Contraindication prothrombinopenia - hypersensitivity - hypersensitivity to other tsefalosporinovy antibiotics, penicillin With care it is necessary to apply to a ceftazidime or any other component of drug in a renal failure, in digestive tract diseases (including in the anamnesis and in nonspecific ulcer colitis), pregnancies, in the period of a lactation and at newborns, at combination with "loopback" diuretics and aminoglycosides. Medicinal interactions Pharmaceutical it is incompatible with aminoglycosides (a considerable mutual inactivation: at simultaneous use these drugs should be administered in different parts of the body) and Vancomycinum (the deposit depending on concentration is formed, if necessary to administer two drugs through one tube, between their use the systems for in/in introductions should be washed out). At simultaneous use with "loopback" diuretics, aminoglycosides, Vancomycinum, clindamycin the risk of nephrotoxic action increases. Bacteriostatic antibiotics (including chloramphenicol) reduce efficiency of drug. The ceftazidime, as well as other antibiotics, can break intestinal microflora that can lead to decrease in a reabsorption of estrogen and decrease in efficiency of the combined oral hormonal contraceptives. If you take other drugs, consult with the doctor. Special instructions the Patients who had allergic reactions to penicillin in the anamnesis can have hypersensitivity to tsefalosporinovy antibiotics. During treatment it is impossible to use ethanol because of a possibility of emergence of disulfiramopodobny reactions (sudden rush of blood to the person, spastic abdominal pain, nausea, vomiting, a headache, tachycardia, an asthma). At simultaneous introduction of a ceftazidime in a high dose with nephrotoxic drugs, such as aminoglycosides and diuretics (furosemide), it is necessary to control function of kidneys. At some patients the pseudomembranous colitis caused by the toxins produced by Clostridium difficile can develop in time or after use of a ceftazidime. In this case stop treatment and carry out the corresponding therapy. Drug can interfere with synthesis of vitamin K owing to suppression of indestinal flora that can cause decrease in level of blood-coagulation factors, dependent on vitamin K, and lead to a prothrombinopenia and bleeding. Prescribing of vitamin K eliminates a prothrombinopenia. Risk of developing bleedings is highest at patients with a severe disease, at patients with an abnormal liver function, at the elderly and weakened patients, at persons with poor nutrition. With care. A renal failure, the neonatality period, colitis in the anamnesis, patients with a sprue (the risk of decrease in prothrombin activity, especially at persons with the profound renal and/or liver failure is increased), bleedings in the anamnesis, a concomitant use with loopback diuretics, aminoglycosides. Pregnancy and in the period of a lactation. At pregnancy, drug is used only if the expected advantage of treatment for mother exceeds potential risk for a fruit. In need of use of drug in the period of a lactation for the period of treatment it is necessary to stop breastfeeding. The feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms Should be careful during the driving of the car and occupations other potentially dangerous types of activity requiring special attention and speed of psychomotor reactions. Overdose Symptoms: neurologic complications with development of encephalopathy, spasms and a coma. Treatment: symptomatic, in case of a renal failure - peritoneal dialysis or a hemodialysis. A form of release and packing of 0.5 g, 1.0 g of active agent in bottles glass with a capacity of 10 ml, corked by rubber bungs, pressed out by caps aluminum. 10 bottles with the instruction for medical use in the state and Russian languages in a box of cardboard. From 1 to 50 bottles and 1-5 instructions for medical use in the state and Russian languages in a box of cardboard. To Store storage conditions in the place protected from light at a temperature not above 25 °C. To store out of children's reach! 2 years not to apply a period of storage after an expiration date! Prescription status According to the prescription JSC Krasfarm Producer, Russia the Owner of the registration certificate of JSC Krasfarm, Russia accepts Claims from consumers the producer, the owner of the registration certificate: JSC Krasfarm, Russia. 660042, Krasnoyarsk, to st. is 60 years of October, 2.
To develop
To develop