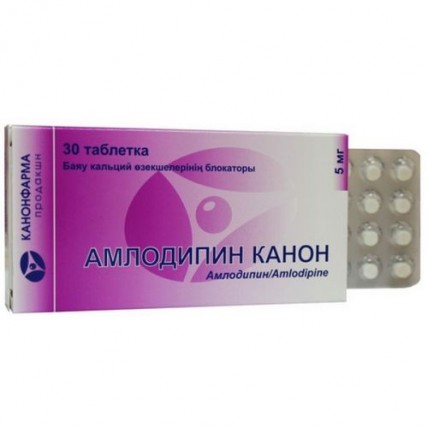
Canon Amlodipine 5 mg (30 tablets)
- $5.30
The instruction for medical use of CANON AMLODIPIN medicine the Trade name Amlodipin Kanon the International unlicensed name Amlodipin Lekarstvennaya a form of the Tablet of 5 mg and 10 mg Structure One tablet contains active agent - an amlodipin of the besilat of 6.93 mg or 13.86 mg, in terms of amlodipin 5 mg or 10 mg, excipients: calcium stearate, potato starch, lactoses monohydrate, magnesium stearate, cellulose microcrystalline. The description of the Tablet of a ploskotsilindrichesky form, white or almost white color, with risky and a facet. Pharmacotherapeutic group Blockers of slow calcium channels. Blockers of slow calcium channels selection. Dihydropyridinic derivatives. Amlodipin. The ATX C08CA01 code the Pharmacological Pharmacokinetics Later properties of intake is slowly absorbed from digestive tract (90%) irrespective of meal. The maximum concentration of drug in blood in 6-12 hours. Concentration of stable balance of drug in blood plasma is reached in 7-8 days after its constant reception. Distribution volume - about 20 l/kg. Bioavailability - 60-65%. Communication with proteins of blood plasma - more than 95%. Elimination half-life of drug - 35-45 hours that allows to appoint it once a day. It is metabolized, generally in a liver with formation of inactive metabolites. Less than 10% of the dose accepted inside are removed in not changed look, about 60% are excreted by kidneys in the form of inactive metabolites, in a renal failure - does not change, 20-25% are removed in the form of metabolites with bile and through intestines and also with breast milk. Gets through a blood-brain barrier. Elimination half-life at patients with arterial hypertension - 48 hours, at elderly patients increases till 65 o'clock, in a liver failure - till 60 o'clock. At a hemodialysis is not removed. Amlodipin's pharmacodynamics - derivative dihydropyridine. Possesses antihypertensive, anti-anginal action. Blocks receipt of calcium ions through cellular membranes in smooth muscle cells of a myocardium and vessels. The mechanism of hypotensive action is caused by the direct weakening impact on unstriated muscles of vessels. The anti-anginal effect of drug is caused by ability to expand peripheral arterioles that leads to decrease in the general peripheric vascular resistance. Reduction of load of heart leads to decrease in need of a myocardium for oxygen. Under the influence of drug due to expansion of coronary arteries the intake of oxygen in a myocardium (increases especially in vasospastic stenocardia). Has no adverse impact on a metabolism and lipids of blood plasma, possesses anti-atherosclerotic action, increases glomerular filtration rate, possesses weak natriuretic effect. At a diabetic nephropathy does not increase severity of a microalbuminuria. Indications In the medical purposes in quality of mono or complex therapy: - arterial hypertension - stable stenocardia and vasospastic stenocardia (Printsmetal's stenocardia) the Route of administration and doses the Initial dose. In arterial hypertension and stenocardia – 5 mg a day. If necessary it can be increased to the maximum daily dose. The maximum daily dose - 10 mg. The patient with an abnormal liver function as antihypertensive amlodipin appoint with care, in an initial dose of 2.5 mg. Dose adjustment is not required from older persons, but more careful observation is necessary. The course of treatment is established by the doctor for each patient individually. Side effects Frequency: frequent - more than 1%, infrequently - less than 1%, are rare - less than 0.1%, is very rare - less than 0.01%. From a cardiovascular system: often - peripheral hypostases (anklebones and feet), heartbeat, infrequently - excessive decrease in the ABP, orthostatic hypotension, a vasculitis, is rare - development or aggravation of heart failure, is very rare - disturbances of a rhythm (bradycardia, ventricular tachycardia, ventricular premature ventricular contraction, trembling and atrial fibrillation), a myocardial infarction, thorax pain, migraine, rushes of blood to the person. From nervous system: often - a headache, dizziness, increased fatigue, drowsiness, infrequently - the indisposition, a syncope, an asthenia, a hypesthesia, paresthesias, peripheral neuropathy, a tremor, insomnia, emotional lability, unusual dreams, nervousness, a depression, alarm, is rare - spasms, apathy, agitation, is very rare - an ataxy, amnesia, confusion of consciousness. From digestive system: often - nausea, abdominal pain, infrequently - vomiting, change of the mode of defecation (including a constipation, a meteorism), dyspepsia, diarrhea, anorexia, dryness of a mucous membrane of an oral cavity, thirst, it is rare - a hyperplasia of gums, increase in appetite, is very rare - gastritis, pancreatitis, a hyperbilirubinemia, jaundice (usually cholestatic), increase in activity of hepatic transaminases, hepatitis. From bodies of a hemopoiesis: very seldom - a Werlhof's disease, a leukopenia, thrombocytopenia. From an urinogenital system: infrequently - a pollakiuria, painful desires on urination, a nocturia, impotence, it is very rare - a dysuria, a polyuria. From the musculoskeletal system: infrequently - an arthralgia, myotonia, myalgia, a dorsodynia, arthrosis, it is rare - a myasthenia. From a respiratory system: infrequently - short wind, rhinitis, it is very rare - cough. Allergic reactions: infrequently - a skin itching, rash, it is very rare - a Quincke's disease, a multiformny erythema, a small tortoiseshell. Other: infrequently - an alopecia, a ring in ears, a gynecomastia, disturbance of sexual function, increase/decrease in body weight, a disorder of vision, a diplopia, accommodation disturbance, a xerophthalmia, conjunctivitis, eye pain, a food faddism, a fever, nasal bleeding, the increased sweating, it is rare - dermatitis, is very rare - cold clammy sweat, a parosmiya, xanthopathy disturbance, a hyperglycemia. Contraindications - hypersensitivity to an amlodipin and other derivatives of dihydropyridine - collapse, cardiogenic shock - heavy arterial hypotension (systolic arterial blood pressure less than 90 mm Hg.) - unstable stenocardia, except for vasospastic stenocardia - the profound aortal stenosis - hypertensive crisis - deficiency of lactase, a lactose intolerance, glyukozo-galaktozny malabsorption - pregnancy and the period of a lactation - the children's and teenage age up to 18 years (the efficiency and safety are not established) Medicinal interactions Is compatible to antibiotics, hypoglycemic drugs for intake. Inhibitors of microsomal oxidation increase concentration of an amlodipin in blood plasma, increasing risk of development of side effects, and inductors of microsomal enzymes of a liver - reduce. Clinically significant interaction with non-steroidal anti-inflammatory drugs, especially indometacin is not noted. Thiazide and loopback diuretics, beta blockers, verapamil, inhibitors of the APF angiotensin-converting enzyme and nitrates strengthen anti-anginal and hypotensive action. Alfa1-adrenoblokatory, neuroleptics, izofluran – enhance hypotensive effect. The hypotensive effect is weakened by sympathomimetics and estrogen. At combined use with the antiarrhytmic drugs causing lengthening of an interval of QT (Amiodaronum, quinidine) the expressiveness of negative inotropic effect can increase. At combined use with drugs of lithium the manifestation of neurotoxicity (nausea, vomiting, diarrhea, an ataxy, a tremor, sonitus) is possible. Amlodipin Kanon does not influence pharmacokinetic parameters of digoxin, warfarin, Phenytoinum, cyclosporine. Drugs of calcium can reduce effect of the blockers of slow calcium channels (BSCC). Antiviral drugs (ritonavir) increase plasma concentration of BMKK, including, an amlodipina. Ethanol (alkogolsoderzhashchy drinks): at single and repeated use in a dose of 10 mg amlodipin has no significant effect on ethanol pharmacokinetics. Single dose of the antacids containing aluminum/magnesium do not influence pharmacokinetics of an amlodipin. Sindenafil (Viagra): single dose of 100 mg of a sindenafil does not influence pharmacokinetics of an amlodipin. Atorvastatin: repeated use of an amlodipin in a dose of 10 mg and an atorvastatina in a dose of 80 mg is not followed by significant changes in pharmacokinetics of an atorvastatin. Special instructions it Is applied with care at: - an aortal stenosis, - chronic heart failure not of the ischemic nature (the III-IV class on NYHA), - a liver failure, - an acute myocardial infarction (and within 1 month after it), - advanced age. As well as at use of other blockers of slow calcium channels, patients need to be careful against the background of reception of an amlodipin with a sick sinus syndrome, a mitral stenosis, a hypertrophic subaortic stenosis, arterial hypotension. During treatment the control of body weight and a diet, especially sodium consumption, maintenance of hygiene of teeth and frequentation of the stomatologist (prevention of morbidity, bleeding and hyperplasia of gums) is necessary. It is necessary to avoid sharp drug withdrawal because of risk of deterioration in a course of stenocardia. Amlodipin Kanon does not affect plasma potassium concentrations, glucose, triglycerides, the general cholesterol, lipoproteins of low density, uric acid, creatinine, urea nitrogen. The feature of influence of medicine on ability to run the vehicle or other potentially dangerous mechanisms was not messages about influence of the drug Amlodipin Kanon on control of motor transport or work with mechanisms. Nevertheless, some patients, mainly in an initiation of treatment, can have a drowsiness and dizziness. At their emergence it is necessary to be careful at control of motor transport and occupation other potentially dangerous types of activity demanding the increased concentration of attention and speed of psychomotor reactions. Overdose Symptoms: profound lowering of arterial pressure, tachycardia, excessive peripheral vazodilatation. Treatment: gastric lavage, prescribing of activated carbon, maintenance of function of a cardiovascular system, monitoring of indicators of cardiac performance and lungs, control of volume of the circulating blood and a diuresis. For restoration of a tone of vessels - use of vasoconstrictive drugs (in the absence of contraindications to their use), for elimination of blockade of calcium channels - intravenous administration of a gluconate of calcium. The hemodialysis is not effective. The form of release and packing On 10 or 30 tablets place in blister strip packaging from a film of the polyvinylchloride and printing aluminum foil varnished. On 3, 6 blister strip packagings on 10 tablets or on 1, 2 blister strip packagings on 30 tablets together with the instruction for medical use in the state and Russian languages place in a pack from cardboard. To Store storage conditions in the dry, protected from light place at a temperature not above 25 °C. To store out of children's reach! 3 years not to use a period of storage after an expiration date. Prescription status According to the prescription CJSC Kanonfarm production Producer, Russian Federation, 141100, Moscow region, Shchyolkovo, Zarechnaya St., 105 Ph.: +7 (495) 797-99-54, fax: +7 (495) 797-96-63 E-mail: www.canonpharma.ru the Name and the country of the owner of the registration certificate of CJSC Kanonfarm production, the Russian Federation the Address of the organization of the accepting claim from the consumer on quality of drug in the territory of the Republic of Kazakhstan: SP N.A. Nesterenko., Republic of Kazakhstan, 050000, Almaty, Furmanova, 128, office 16, ph.: +7 727 2796659, e-mail:
To Develop ip_n_nesterenko@list.ru
To Develop ip_n_nesterenko@list.ru