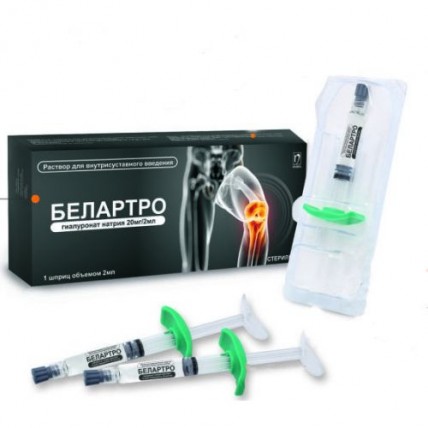
Belartro 20 mg/2 ml 2 ml for intra-articular injections in the syringe
- $152.90
Sku:
452a44e1f875
Brand:
Humedix Co., Ltd. (Korea)
The instruction for use of a product of medical purpose the Name of a product of medical purpose of BELARTRO, an implant vyazkoelastichny for intra articulate introduction Structure and the description of a product In 1 ml contains in the 2 ml syringe: 10 mg of sodium hyaluronate and also sodium chloride, sodium hydrophosphate, sodium dihydrophosphate, water for BELARTRO injections - the sterile transparent solution containing the sodium hyaluronate in the physiological buffer which is previously filled in the 2 ml syringe for single use and packed into the blister packing. On 1 blister packing together with the instruction for use in the state and Russian languages place in a pack from cardboard. The scope For treatment in pains and restriction of mobility of joints (knee joint osteoarthritis) Sodium hyaluronate (sodium salt of hyaluronic acid) belongs to group of rare substances which are identical at all living organisms. It is the natural polysaccharide which is present at all body tissues especially in high concentrations as a part of synovial fluid and skin. BELARTRO is split in an organism in the same metabolic way, as well as endogenous hyaluronic acid. Hyaluronic acid has mechanical effect which allows to apply it during inflammatory processes as replacement of synovial fluid which quality worsened. Thus, by means of restoration of physical characteristics of the intra articulate environment, the mobility of joints can improve and also pain in an osteoarthritis of a knee and a periarthritis of a shoulder can be weakened. Other potential mechanism of action can be connected with the action including synthesis of hyaluronic acid with a high molecular weight which is similar to 'normal' hyaluronic acid. Without this incentive, a molecule of hyaluronic acid which are synthesized by these sinoviotsita have the molecular weight which is lower than normal. This last mechanism of action can explain long clinical effect of sodium of hyalrunate. Intra articulate introduction of BELARTRO improves function of joints, stops the pain caused by an osteoarthritis of knee and humeral joints, BELARTRO improves mobility of joints and normalizes composition of synovial fluid. BELARTRO is also shown for use concerning a trapezoid and metacarpal joint for patients rizoartrozy. The route of administration Is recommended one injection (2 ml) in a cavity of the affected joint once a week, a course from 3 injections by a standard technique. It is at the same time possible to carry out injections and in other joints. However it is necessary to make the decision on use only of one or other method for an individual patient, in this context, it is easier to carry out introduction under endoscopic observation. In all cases, it is necessary to adjust a dose depending on weight of symptoms. As well as any similar invasive articulate procedure, is recommended to patients to avoid any physical activity after introduction of an intra articulate injection, having completed a restoration course within several days. The introduction technique As is entered by BELARTRO into intra articulate space, all process has to be carried out in strictly sterile conditions and has to be entered only by the qualified doctor. The place of an injection before introduction needs to be wiped with alcohol or other corresponding antiseptic solution. Considering degree of viscosity of drug, it is recommended to apply needles of sizes 22-23G. Before an injection of BELARTRO it is necessary to eliminate exudate if it is. For elimination of exudate and an injection of BELARTRO it is necessary to use the same needle. To enter BELARTRO only into a joint crack once. After completion of a session of treatment it is necessary to throw out the syringe, a needle and any other unused material in garbage. Removal of the local secondary phenomena, such as pains, caumesthesia, reddening and swelling on a joint, requires imposing of ice on a joint during five — ten minutes. Precautionary measures - not to enter intravenously - it is necessary to be careful to avoid introduction of an injection of BELARTRO to blood vessels or surrounding fabrics, not to enter by an extraarticular method or in synovial fabric or the capsule - not to apply if to an injection considerable intra articulate exudate took place - not to mix BELARTRO with other injection materials - if the product was stored in the fridge (look in storage conditions), then it is necessary to bring it to room temperature before use - apply a product only once and at once after its opening. Do not use a product if in advance filled syringe or the blister packing are damaged - you will throw out any unused part of the drug Incompatibility Avoid of contact with disinfectants, such as quaternary ammonium salts, including a benzalkoniya chloride, or with chlorhexidiny as they can cause loss of drug in a deposit. Contraindications of BELARTRO are forbidden to be entered: - to patients who have an allergy to the drugs containing hyaluronic acid - at presence of a skin disease or infection near the place of an injection - in an infection or strong inflammation of a joint of a knee As pregnant women and children have no clinical data on use of BELARTRO, it is not recommended to use to children, pregnant women and nursing mothers Side effects: Seldom - hypostases, reddening, feeling of heat and weight - the non-constant (passing) pain, swelling - knee exudate where it was made injections the Above-stated symptoms have passing character and usually disappear later 24 h. In certain cases exudate can be considerable and lead to pronounced pain. It is important to cancel treatment and to analyze solution for an exception of an infection or a crystal arthropathy. These reactions usually decrease after several days. Very seldom - intra articulate infections - - manifestations of skin rash (urticaria, an itching) Storage conditions to Store shock In isolated cases in the place protected from light, at a temperature from +2 to +25 °C. Not to freeze. To store out of children's reach. A period of storage 2 years in original packing. Not to apply after expiry date. Name, country, legal address of the manufacturing organization: Humedix To., Ltd., Korea, 17, Bio Valley 2-ro, Jecheon-si, Chungcheonbuk-do, 390-250 Owner of the registration certificate of JSC Nobel Almatinskaya Pharmatsevticheskaya Fabrika the Republic of Kazakhstan the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods): JSC Nobel Almatinskaya Pharmatsevticheskaya Fabrika