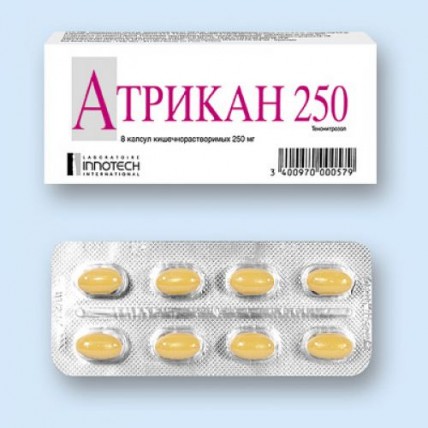
Atrikan 8's 250 mg capsules
- $14.20
Out Of Stock
Structure and form of release
of the Capsule kishechnorastvorimy soft gelatinous, opaque, an ovoid form, yellowy-brown color, about 7.5 mm x 14 mm in size, contents of capsules - gray-brown homogeneous oily mass.
1 caps.
tenonitrozol
the 250th mgprochy ingredients: peanut butter, the oil of soybeans hydrogenated, soy lecithin, gelatin, sorbitol and sorbitan solution, glitserol, gipromelloza phthalate, dibutyl phthalate, the titan dioxide (E171), ferrous oxide yellow (E172).8 of piece - blisters (1) - packs cardboard.
Registration number No.: caps. kishechnorastvorimy 250 mg: 8 pieces - P No. 014806/01-2003 18.02.03
Pharmacological action
Antiprotozoan drug. It is active concerning Trichomonas vaginalis. Has also disulfiramopodobny effect.
Indications
– trichomoniasis of an urinogenital system.
The dosing mode
Drug is appointed inside on 250 mg of 2 times/days (in the morning and in the evening) during meal within 4 days. It is not recommended to exceed a daily dose. In case of the admission in reception of one or several doses it is necessary to resume administration of drug in a usual dose.
Side effect
from digestive system: sometimes - nausea, feeling of weight in epigastriums, loss of appetite. Other: allergic reactions.
Contraindications
– an acute and chronic liver failure, – children's age, – hypersensitivity to a tenonitrozol and other components of drug.
Pregnancy and a lactation
It is desirable not to use drug at pregnancy. Do not recommend to use drug in the period of a lactation (breastfeeding) since tenonitrozol it is allocated with breast milk.
The special
instructions Drug use for treatment of trichomoniasis only at adults. During treatment it is necessary to avoid alcohol intake and the medicines containing ethanol. It is not necessary to appoint drug to the persons not capable to abstain from alcohol intake for the period of treatment. Performing simultaneous treatment of sexual partners is necessary. It is not recommended to carry contact lenses during the Atrikan drug treatment 250 (possibly penetration of active agent into eye liquid). At Atrikan's use 250 coloring of scleras and urine in yellow color is possible. The patient has to be informed on need to report to the doctor about existence in the anamnesis of episodes of change of number of leukocytes in peripheral blood against the background of intake of other medicines.
Overdose
Now about cases of overdose of the drug Atrikan 250 it was not reported.
Medicinal interaction
Should be considered that during use of drug the intake of ethanol is contraindicated.
Conditions and periods of storage
List B. Drug should be stored in the place, dry, inaccessible for children, at a temperature not above 30 °C. An expiration date - 3 years. Prescription status from aptekpreparat it is released on the prescription.
of the Capsule kishechnorastvorimy soft gelatinous, opaque, an ovoid form, yellowy-brown color, about 7.5 mm x 14 mm in size, contents of capsules - gray-brown homogeneous oily mass.
1 caps.
tenonitrozol
the 250th mgprochy ingredients: peanut butter, the oil of soybeans hydrogenated, soy lecithin, gelatin, sorbitol and sorbitan solution, glitserol, gipromelloza phthalate, dibutyl phthalate, the titan dioxide (E171), ferrous oxide yellow (E172).8 of piece - blisters (1) - packs cardboard.
Registration number No.: caps. kishechnorastvorimy 250 mg: 8 pieces - P No. 014806/01-2003 18.02.03
Pharmacological action
Antiprotozoan drug. It is active concerning Trichomonas vaginalis. Has also disulfiramopodobny effect.
Indications
– trichomoniasis of an urinogenital system.
The dosing mode
Drug is appointed inside on 250 mg of 2 times/days (in the morning and in the evening) during meal within 4 days. It is not recommended to exceed a daily dose. In case of the admission in reception of one or several doses it is necessary to resume administration of drug in a usual dose.
Side effect
from digestive system: sometimes - nausea, feeling of weight in epigastriums, loss of appetite. Other: allergic reactions.
Contraindications
– an acute and chronic liver failure, – children's age, – hypersensitivity to a tenonitrozol and other components of drug.
Pregnancy and a lactation
It is desirable not to use drug at pregnancy. Do not recommend to use drug in the period of a lactation (breastfeeding) since tenonitrozol it is allocated with breast milk.
The special
instructions Drug use for treatment of trichomoniasis only at adults. During treatment it is necessary to avoid alcohol intake and the medicines containing ethanol. It is not necessary to appoint drug to the persons not capable to abstain from alcohol intake for the period of treatment. Performing simultaneous treatment of sexual partners is necessary. It is not recommended to carry contact lenses during the Atrikan drug treatment 250 (possibly penetration of active agent into eye liquid). At Atrikan's use 250 coloring of scleras and urine in yellow color is possible. The patient has to be informed on need to report to the doctor about existence in the anamnesis of episodes of change of number of leukocytes in peripheral blood against the background of intake of other medicines.
Overdose
Now about cases of overdose of the drug Atrikan 250 it was not reported.
Medicinal interaction
Should be considered that during use of drug the intake of ethanol is contraindicated.
Conditions and periods of storage
List B. Drug should be stored in the place, dry, inaccessible for children, at a temperature not above 30 °C. An expiration date - 3 years. Prescription status from aptekpreparat it is released on the prescription.