The instruction for medical use
of ARTRA® medicine
the Trade name
of Artra®
the International unlicensed name
Is not present
the Dosage form
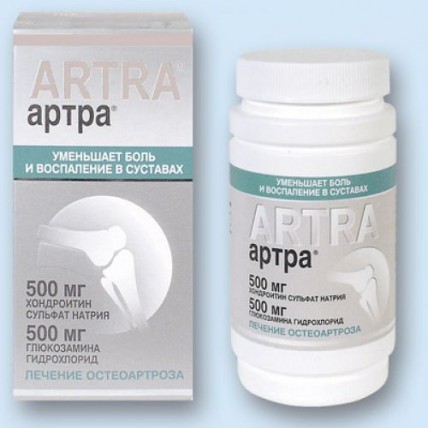
of the Tablet, film coated
Structure
One tablet contains
active agents:
a glycosamine a hydrochloride
– 500 mg
chondroitin sodium sulfate
– 500 mg,
excipients: -
ShchESTVOkaltsiya phosphate disubstituted, microcrystalline cellulose, sodium of a kroskarmelloz, stearic acid, magnesium stearate,
structure of a cover: hydroxypropyl methyl cellulose, titan dioxide
(E 171), triacetin.
The description
the Biconvex tablets of an oval form film coated from color, white to white with a yellowish shade, with impregnations, with an engraving of ARTRA on the one side of a tablet, with a specific smell.
Pharmacotherapeutic group
Other drugs for treatment of diseases of a musculoskeletal system.
The code of automatic telephone exchange M09AX
the Pharmacological
Pharmacokinetics Bioavailability properties of a glycosamine at oral administration of 25%. It is distributed in fabrics: the highest concentrations are found in a liver, kidneys and an articulate cartilage. About 30% of the accepted dose are long persistirut in bone and muscle tissue.
It is excreted mainly with urine in an invariable look, partially with a stake.
Т½ – 68 h. Bioavailability sulfate chondroitin about 13%.
The pharmacodynamics
of Artra® stimulates regeneration of cartilaginous tissue. The glycosamine and chondroitin sodium sulfate take part in biosynthesis of connective tissue, promoting prevention of processes of destruction of a cartilage and stimulating fabric regeneration. Introduction of an exogenous glycosamine strengthens development of a cartilaginous matrix and provides nonspecific protection, including against NPVP and glucocorticosteroids (GKS). Drug possesses moderate anti-inflammatory action.
Chondroitin sodium sulfate irrespective of whether it is soaked up in an intact form or in the form of separate components, serves as additional substrate for formation of a healthy cartilaginous matrix. Stimulates formation of proteoglycans and II collagen and also protects a cartilaginous matrix from zymolysis (by suppression of activity of hyaluronidase) and from the damaging action of free radicals, maintains viscosity of synovial fluid, stimulates mechanisms of a reparation of a cartilage and suppresses activity of those enzymes (elastase, hyaluronidase) which split a cartilage. At treatment of an osteoarthritis facilitates symptoms of a disease and reduces the need for NPVP.
Indications
- an osteoarthrosis of peripheral joints and a backbone.
The route of administration and doses
to Adults and children are more senior than 15 years on 1 tablet 2 times a day within the three first weeks, on 1 tablet within the next weeks and months once a day. The steady medical effect is reached at administration of drug not less than 6 months.
Side effects
Seldom
- pains in epigastriums, a meteorism, diarrhea, a constipation
- dizziness
- skin allergic reactions
In some cases
-
the Contraindication sleep disorder
- hypersensitivity to drug components
- the profound renal failure
- children's age up to 15 years
- pregnancy and the period of a lactation
Medicinal interactions
At combined use is possible strengthening of effect of anticoagulants and antiagregant.
Артра® increases absorption of tetracyclines, reduces effect of semi-synthetic penicillin.
Drug is compatible to glucocorticoid drugs.
Special instructions
With care appoint drug in bleedings or tendency to bleedings, bronchial asthma, diabetes.
The feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
does not influence.
Overdose
Symptoms: cases of overdose are unknown.
Treatment: gastric lavage, symptomatic therapy.
Form of release and packing
On 30, 60, 100 or 120 tablets film coated in a bottle polymeric white color, corked by the screwing-up cover from the same material and the safety valve from a foil.
On a bottle paste the label, cover with film polyethylene and together with the instruction for medical use place in a pack from cardboard.
To Store storage conditions at a temperature of 10 - 30 0C, in the dry place.
To store out of children's reach!
Not to use a period of storage of 5 years after the expiry date specified on packing.
Prescription status
Without prescription
Unipharm Producer, INK., the USA
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods)
Representation Unipharm, INK. (USA) in
Republic of Kazakhstan Almaty, Nauryzbay St. of the batyr 17, office 101
Phone number: 8 (727) 244-50-04
Fax number: 8 (727) 244-50-06
E-mail address: info@unipharm.kz
of ARTRA® medicine
the Trade name
of Artra®
the International unlicensed name
Is not present
the Dosage form
of the Tablet, film coated
Structure
One tablet contains
active agents:
a glycosamine a hydrochloride
– 500 mg
chondroitin sodium sulfate
– 500 mg,
excipients: -
ShchESTVOkaltsiya phosphate disubstituted, microcrystalline cellulose, sodium of a kroskarmelloz, stearic acid, magnesium stearate,
structure of a cover: hydroxypropyl methyl cellulose, titan dioxide
(E 171), triacetin.
The description
the Biconvex tablets of an oval form film coated from color, white to white with a yellowish shade, with impregnations, with an engraving of ARTRA on the one side of a tablet, with a specific smell.
Pharmacotherapeutic group
Other drugs for treatment of diseases of a musculoskeletal system.
The code of automatic telephone exchange M09AX
the Pharmacological
Pharmacokinetics Bioavailability properties of a glycosamine at oral administration of 25%. It is distributed in fabrics: the highest concentrations are found in a liver, kidneys and an articulate cartilage. About 30% of the accepted dose are long persistirut in bone and muscle tissue.
It is excreted mainly with urine in an invariable look, partially with a stake.
Т½ – 68 h. Bioavailability sulfate chondroitin about 13%.
The pharmacodynamics
of Artra® stimulates regeneration of cartilaginous tissue. The glycosamine and chondroitin sodium sulfate take part in biosynthesis of connective tissue, promoting prevention of processes of destruction of a cartilage and stimulating fabric regeneration. Introduction of an exogenous glycosamine strengthens development of a cartilaginous matrix and provides nonspecific protection, including against NPVP and glucocorticosteroids (GKS). Drug possesses moderate anti-inflammatory action.
Chondroitin sodium sulfate irrespective of whether it is soaked up in an intact form or in the form of separate components, serves as additional substrate for formation of a healthy cartilaginous matrix. Stimulates formation of proteoglycans and II collagen and also protects a cartilaginous matrix from zymolysis (by suppression of activity of hyaluronidase) and from the damaging action of free radicals, maintains viscosity of synovial fluid, stimulates mechanisms of a reparation of a cartilage and suppresses activity of those enzymes (elastase, hyaluronidase) which split a cartilage. At treatment of an osteoarthritis facilitates symptoms of a disease and reduces the need for NPVP.
Indications
- an osteoarthrosis of peripheral joints and a backbone.
The route of administration and doses
to Adults and children are more senior than 15 years on 1 tablet 2 times a day within the three first weeks, on 1 tablet within the next weeks and months once a day. The steady medical effect is reached at administration of drug not less than 6 months.
Side effects
Seldom
- pains in epigastriums, a meteorism, diarrhea, a constipation
- dizziness
- skin allergic reactions
In some cases
-
the Contraindication sleep disorder
- hypersensitivity to drug components
- the profound renal failure
- children's age up to 15 years
- pregnancy and the period of a lactation
Medicinal interactions
At combined use is possible strengthening of effect of anticoagulants and antiagregant.
Артра® increases absorption of tetracyclines, reduces effect of semi-synthetic penicillin.
Drug is compatible to glucocorticoid drugs.
Special instructions
With care appoint drug in bleedings or tendency to bleedings, bronchial asthma, diabetes.
The feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
does not influence.
Overdose
Symptoms: cases of overdose are unknown.
Treatment: gastric lavage, symptomatic therapy.
Form of release and packing
On 30, 60, 100 or 120 tablets film coated in a bottle polymeric white color, corked by the screwing-up cover from the same material and the safety valve from a foil.
On a bottle paste the label, cover with film polyethylene and together with the instruction for medical use place in a pack from cardboard.
To Store storage conditions at a temperature of 10 - 30 0C, in the dry place.
To store out of children's reach!
Not to use a period of storage of 5 years after the expiry date specified on packing.
Prescription status
Without prescription
Unipharm Producer, INK., the USA
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods)
Representation Unipharm, INK. (USA) in
Republic of Kazakhstan Almaty, Nauryzbay St. of the batyr 17, office 101
Phone number: 8 (727) 244-50-04
Fax number: 8 (727) 244-50-06
E-mail address: info@unipharm.kz