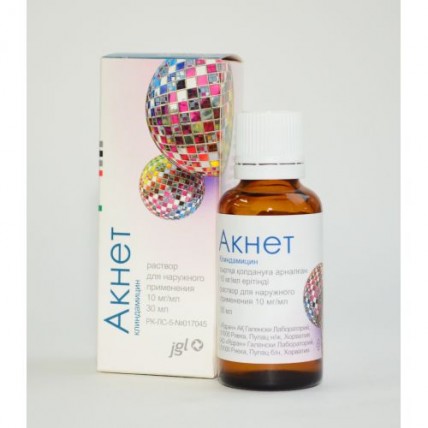
Aknet 10 mg / ml, 30 ml of solution for external use in vial
- $19.10
Out Of Stock
The instruction for medical use
of medicine
AKHET
the Trade name
of Aknet Mezhdunarodnoye the unlicensed
name Clindamycin Dosage Form Solution for external use of 10 mg/ml 30 ml
Structure
of 1 ml of solution contains
active agent of clindamycin a hydrochloride of 11.4 mg.
excipients: alcohol of 96%, propylene glycol, the water purified.
The description
Colourless transparent solution with an alcohol smell.
Pharmacotherapeutic group
Antimicrobial drugs for treatment of acne rash.
The code of automatic telephone exchange D10AF01
the Pharmacological
Pharmacokinetics Insignificant Part properties of clindamycin is exposed to system absorption and further is output from an organism.
A pharmacodynamics
the Antibiotic from group of linkozamid for topical administration in dermatology. Possesses a broad spectrum of activity, contacts 50S a subunit of a ribosomalny membrane and suppresses synthesis of protein in a microbic cell. Bactericidal action has bacteriostatic action, in higher concentrations concerning a number of gram-positive cocci.
Drug is active concerning the bacteria which are most often causing acne rash: Staphylococcus epidermidis (including, producing and not producing penicillinase) and also Propionibacterium acnes. Besides, drug is effective concerning Streptococcus spp. (excepting Enterococcus faecalis), Streptococcus pneumoniae, Corynebacterium diphtheriae, Mycoplasma spp., anaerobic and mikroaerofilny gram-positive cocci (including Peptococcus spp. and Peptostreptococcus spp.), Clostridium perfringens, Clostridium tetani, Bacteroides spp. (including Bacteroides fragilis and Prevotella melaninogenica), Fusobacterium spp., eubakterium and Actinomyces israelii.
Cross clindamycin and lincomycin resistance of microorganisms is noted.
Indications
- the inflamed papules and pustules in acne rash
- a rozatsea
- a sycosis
- perioral dermatitis
the Route of administration and doses
to Adults and children since 12 years.
A thin film of solution is applied on affected areas of the washed-up and cleaned skin 2 times a day, in the morning and in the evening. At most of patients the visible improvement occurs within 2 weeks (the maximum efficiency is reached in 4-5 weeks).
Side effects
- dryness, local irritation (in the site of application), contact dermatitis.
At system absorption there is a likelihood of development of disturbances of the GIT functions (abdominal pain, slight diarrhea), in rare instances pseudomembranous colitis.
The contraindication
- hypersensitivity to the basic and auxiliary components of drug
- children's age up to 12 years
Medicinal interactions
is not recommended simultaneous use of clindamycin of a hydrochloride with other drugs for treatment of acne rash containing the peeling, softening and abrasive substances (for example, benzoyl peroxide, tretinoin, Resorcinolum, salicylic acid, sulfur) because of possible cumulative irritant action on skin.
It is not necessary to use clindamycin and erythromycin at the same time.
Special instructions
It is necessary to avoid hit of drug in eyes or on a mucous membrane of an oral cavity and a nose.
Pregnancy and the period of a lactation
Local use of drug at pregnant women is justified only according to strict indications.
To the feeding women drug is admissible to be applied, only if its use is necessary, at the same time it is necessary to minimize amount of the applied drug and treatment duration.
Features of influence on ability to run motor transport or other mechanisms
the Overdose does not influence
the Form of release and packing
On 30 ml of solution in a bottle from dark glass with the screw-on cover and the doser is not revealed. The bottle together with the instruction for use in the state and Russian languages is placed in a cardboard pack.
To Store storage conditions in the dry, protected from light place, at a temperature not over 25C
to Store out of children's reach!
2 years
not to apply a period of storage after the expiration date specified on packing.
Prescription status
Without prescription
Producer JSC Yadran Galenski Laboratory 51000, Pulats/N, Rijeka, Croatia.
To send claims for quality of drug to Representative office of JSC Yadran Galenski Laboratory in Republic of Kazakhstan, Almaty, Luganskogo St. 54, a cat.9, ph. +7 272 58-27-54, +7 272 58-37-66, to www.jadran.ru
of medicine
AKHET
the Trade name
of Aknet Mezhdunarodnoye the unlicensed
name Clindamycin Dosage Form Solution for external use of 10 mg/ml 30 ml
Structure
of 1 ml of solution contains
active agent of clindamycin a hydrochloride of 11.4 mg.
excipients: alcohol of 96%, propylene glycol, the water purified.
The description
Colourless transparent solution with an alcohol smell.
Pharmacotherapeutic group
Antimicrobial drugs for treatment of acne rash.
The code of automatic telephone exchange D10AF01
the Pharmacological
Pharmacokinetics Insignificant Part properties of clindamycin is exposed to system absorption and further is output from an organism.
A pharmacodynamics
the Antibiotic from group of linkozamid for topical administration in dermatology. Possesses a broad spectrum of activity, contacts 50S a subunit of a ribosomalny membrane and suppresses synthesis of protein in a microbic cell. Bactericidal action has bacteriostatic action, in higher concentrations concerning a number of gram-positive cocci.
Drug is active concerning the bacteria which are most often causing acne rash: Staphylococcus epidermidis (including, producing and not producing penicillinase) and also Propionibacterium acnes. Besides, drug is effective concerning Streptococcus spp. (excepting Enterococcus faecalis), Streptococcus pneumoniae, Corynebacterium diphtheriae, Mycoplasma spp., anaerobic and mikroaerofilny gram-positive cocci (including Peptococcus spp. and Peptostreptococcus spp.), Clostridium perfringens, Clostridium tetani, Bacteroides spp. (including Bacteroides fragilis and Prevotella melaninogenica), Fusobacterium spp., eubakterium and Actinomyces israelii.
Cross clindamycin and lincomycin resistance of microorganisms is noted.
Indications
- the inflamed papules and pustules in acne rash
- a rozatsea
- a sycosis
- perioral dermatitis
the Route of administration and doses
to Adults and children since 12 years.
A thin film of solution is applied on affected areas of the washed-up and cleaned skin 2 times a day, in the morning and in the evening. At most of patients the visible improvement occurs within 2 weeks (the maximum efficiency is reached in 4-5 weeks).
Side effects
- dryness, local irritation (in the site of application), contact dermatitis.
At system absorption there is a likelihood of development of disturbances of the GIT functions (abdominal pain, slight diarrhea), in rare instances pseudomembranous colitis.
The contraindication
- hypersensitivity to the basic and auxiliary components of drug
- children's age up to 12 years
Medicinal interactions
is not recommended simultaneous use of clindamycin of a hydrochloride with other drugs for treatment of acne rash containing the peeling, softening and abrasive substances (for example, benzoyl peroxide, tretinoin, Resorcinolum, salicylic acid, sulfur) because of possible cumulative irritant action on skin.
It is not necessary to use clindamycin and erythromycin at the same time.
Special instructions
It is necessary to avoid hit of drug in eyes or on a mucous membrane of an oral cavity and a nose.
Pregnancy and the period of a lactation
Local use of drug at pregnant women is justified only according to strict indications.
To the feeding women drug is admissible to be applied, only if its use is necessary, at the same time it is necessary to minimize amount of the applied drug and treatment duration.
Features of influence on ability to run motor transport or other mechanisms
the Overdose does not influence
the Form of release and packing
On 30 ml of solution in a bottle from dark glass with the screw-on cover and the doser is not revealed. The bottle together with the instruction for use in the state and Russian languages is placed in a cardboard pack.
To Store storage conditions in the dry, protected from light place, at a temperature not over 25C
to Store out of children's reach!
2 years
not to apply a period of storage after the expiration date specified on packing.
Prescription status
Without prescription
Producer JSC Yadran Galenski Laboratory 51000, Pulats/N, Rijeka, Croatia.
To send claims for quality of drug to Representative office of JSC Yadran Galenski Laboratory in Republic of Kazakhstan, Almaty, Luganskogo St. 54, a cat.9, ph. +7 272 58-27-54, +7 272 58-37-66, to www.jadran.ru