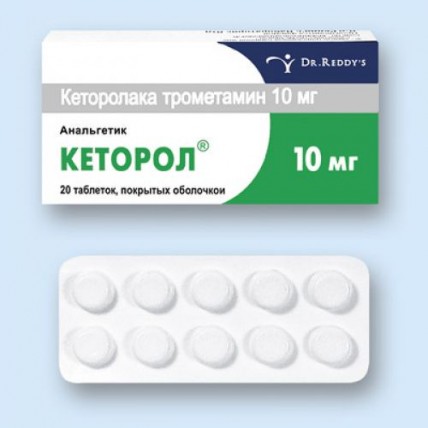
Ketorol 20s 10 mg coated tablets
- $4.00
The instruction for medical use
of Ketorol Torgovoye medicine a name
Ketorol
Mezhdunarodnoye the unlicensed
name Ketorolak Lekarstvennaya
the Tablet form, film coated 10 mg
Structure
One tablet contains
active agent ketorolak a trometamina of 10 mg
excipients:
cellulose microcrystalline (type 102), starch corn prezhelatinizirovanny, starch corn, silicon dioxide anhydrous colloid, magnesium stearate
structure of a cover: Opadri 03K51148 green (hypro methyl cellulose (6cps), the titan E171 dioxide, triacetin/glycerin, gland (III) oxide yellow E172, FD & With No. 1 dye (diamond blue FCF, aluminum varnish of 11-13%)
the Description
of the Tablet of round shape, with a biconvex surface, film coated olive-green color, with S engraving on one party and a smooth surface on other party, diameter (8.20 & plusmn, 0.20) mm and thickness (3.50 & plusmn, 0.20) mm.
Pharmacotherapeutic group
Non-steroidal anti-inflammatory drugs. Derivatives of acetic acid.
The code of automatic telephone exchange M01AB15
the Pharmacological
Pharmacokinetics At properties intake ketorolak is well soaked up from digestive tract. The maximum concentration in blood plasma (0,7 1,1 mkg/ml) is reached in 40 minutes after reception on an empty stomach of a dose of 10 mg. Food rich with fats reduces Cmax and delays its achievement on 1 hour
Ketorol almost completely contacts proteins of plasma (& gt, 99%) at the expense of the small volume of distribution (& lt, 0.3 l/kg).
Time of achievement of equilibrium concentration at oral administration 24 hours when assigning 4 times a day. At intake of 10 mg it makes 0.39 0.79 mkg/ml.
Almost all substance circulating in blood plasma represents ketorolak (96%) or it pharmacological an inactive metabolite r-hydroksiketorolak.
Drug passes through a placental barrier for 10%. In small concentration it is found in breast milk of women. Passes through a blood-brain barrier badly.
Drug is metabolized mainly in a liver, contacting glucuronic acid, and is removed by kidneys. In urine about 92% of the entered drug dose, and 40% - in the form of metabolites, 60% - in the form of not changed substance are found. About 6% of the entered dose are removed with a stake. Metabolites have no essential analgesic activity.
At patients of senile age (65 years are more senior) the elimination half-life of a terminal phase, in comparison with that at persons of young age, increases till 7 o'clock (from 4.3 to 8.6 hours). The general plasma clearance, in comparison with persons of young age, can be reduced on average to 0.019 l/hour/kg.
At defeat of function of kidneys the removal of a ketorolak slows down what lengthening of elimination half-life and decrease in the general plasma clearance in comparison with young healthy volunteers testifies to. Speed of elimination decreases approximately in proportion to degree of a renal failure, except for patients with a heavy renal failure. At such patients the plasma clearance of a ketorolak becomes slightly higher than the damage of kidneys expected for this degree.
A pharmacodynamics
Ketorol the non-steroidal anti-inflammatory drug (NPVS) possessing soothing and anti-inflammatory action. The main mechanism of action of a ketorol, as well as at other NPVS, is shown in its pharmacological effect - inhibition of synthesis of prostaglandins. NPVS are most active on the periphery.
Ketorol has no sedative or anxiolytic effect, does not influence opioid receptors. Ketorol does not possess the oppressing action on a respiratory center and does not strengthen the respiratory depression and sedation caused by opioid analgetika. Ketorol does not cause medicinal dependence. After sharp drug withdrawal the symptoms of cancellation do not arise.
Indications
- stopping of pains in the postoperative period
- stopping of pains in muscles and joints
- a posttraumatic pain syndrome
- renal colic
the Route of administration and doses
Is appointed a short course of treatment and is applied within 7 days, it is not recommended for long-term treatment.
Adults
On 10 mg of each 4 6 hours. The daily dose should not exceed 40 mg.
For the patients accepting ketorol parenterally the general dose of drug at combination therapy should not exceed 90 mg a day.
Side effects
Often (more than 3%)
- a headache, dizziness, drowsiness
- edemas of face, shins, anklebones, fingers, a foot
- increase in body weight
is Less frequent (1 3%)
- skin rash, a purpura
- the increased perspiration
Seldom (less than 1%)
- an acute renal failure, a back pain with or without hamaturia and/or azotemias, a hemolytic uraemic syndrome (hemolytic anemia, a renal failure, thrombocytopenia, a purpura), frequent urination, increase or decrease in volume of urine, nephrite, swell renal genesis
- decrease in hearing, a ring in ears, a disorder of vision
- a bronchospasm or dispnoe, rhinitis, a laryngeal edema
- anemia, an eosinophilia, a leukopenia
- bleeding from a postoperative wound, nasal bleeding, rectal bleeding
- an anaphylaxis or anaphylactoid reactions (discoloration of face skin, skin rash, a small tortoiseshell, an itching of skin, a tachypnea, swell a century, periorbital swelled, an asthma, the complicated breath, weight in a thorax, goose breathing)
- aseptic meningitis (fever, a severe headache, spasms, muscle tension of a neck and/or back), hallucinations, a depression, psychosis
- exfoliative dermatitis (fever with a fever or without it, reddening, consolidation or peeling of skin, swelling and/or morbidity of palatine tonsils), a small tortoiseshell, Stephens-Johnson's syndrome, a Lyell's disease
- a paraglossa, fever.
Contraindications
- hypersensitivity to a ketorol, aspirin or to other NPVS
- aspirinovy asthma, a Quincke's disease
- a hypovolemia
- dehydration
- digestive tract erosive cankers
- round ulcers
- hypocoagulation, including hemophilia
- a liver, renal failure
- a hemorrhagic stroke
- bleedings, including after operation
- hemopoiesis disturbance
- before - and the operational period, because of high risk of bleeding
- chronic pains
- pregnancy and the period of a lactation
- children's and teenage age up to 18 years
the Simultaneous use of a ketorol with acetylsalicylic acid or other NPVS, calcium drugs, glucocorticoids, ethanol, corticotropin can lead Medicinal interactions to formation of ulcers of digestive tract and development of gastrointestinal bleedings.
Combined use with paracetamol increases nephrotoxicity, with a methotrexate gepato- and nephrotoxicity. Co-administration of a ketorol and methotrexate is possible only when using low doses of the last.
Probenetsid reduces plasma clearance and volume of distribution of a ketorol, increases its concentration in blood plasma and extends elimination half-life twice. The concomitant use of a ketorol and a probenetsid is contraindicated.
At combination with opioid analgetics, doses of the last can be significantly reduced.
Hypoglycemic effect of insulin and oral hypoglycemic drugs increases.
Joint appointment with Valproatum of sodium causes disturbance of aggregation of thrombocytes.
Ketorol increases concentration in blood plasma of verapamil and nifedipine.
When assigning with other nefrotoksichny medicines, including with gold drugs, risk of development of nephrotoxicity increases.
The medicines blocking canalicular secretion reduce clearance of a ketorol and increase its concentration in blood plasma.
Ketorol slightly reduces linking of warfarin with proteins. At a concomitant use with furosemide ketorol reduces diuretic effect of furosemide approximately by 20%.
At a concomitant use of a ketorol with drugs of lithium the concentration of lithium in plasma at the expense of inhibition of clearance of lithium kidneys some NPVS increases.
At a concomitant use of a ketorol and not depolarizing muscular relaxants the patients have a short wind.
At reception with antiepileptic drugs (Phenytoinum, carbamazepine) the frequency of attacks amplifies. At a concomitant use with psychotropic drugs (fluoxetine, tiotiksin, to alprazola) patients have hallucinations.
Co-administration and a pentoksifillina can increase risk of developing bleedings.
At simultaneous use with furosemide ketorol reduces diuretic effect of furosemide approximately by 20% therefore patients with heart failure need to appoint drug with care.
At at the same time use with APF inhibitors ketorol increases risk of a renal failure.
Special instructions
As NPVS reduce aggregation of thrombocytes, it is necessary to appoint ketorol with care the patient with disturbance of a coagulant system of blood.
Use for patients from the liver broken by function
At reception of a ketorol is possible increase in level of hepatic transaminases. Ketorol it is necessary to appoint a short course of treatment the patient with liver diseases.
Use for patients with impaired renal function
Ketorol is appointed the patient with impaired renal function with care under control of analyses of urine.
Use for patients of advanced age
At reception of a ketorol the side reactions at patients of advanced age meet more often, purpose of low doses of drug is necessary. The maximum doses should not exceed 60 mg for patients 65 years are more senior.
It is necessary to take the drug with an interval of 6 8 hours.
Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
As at patients when assigning a ketorol develop by-effects from the central nervous system (dizziness, drowsiness) and from sense bodys (decrease in hearing, a ring in ears, a disorder of vision), it is recommended to avoid performance of work, the requiring special attention and fast reaction.
Overdose
Symptoms: abdominal pains, nausea, vomiting, round ulcer of a stomach and duodenum, erosive gastritis, renal failure, metabolic acidosis.
Treatment: gastric lavage, administration of adsorbents (the coal activated), symptomatic treatment.
A form of release and packing
On 10 tablets in blister strip packaging from a film of the polyvinylchloride and printing aluminum foil varnished.
On 2 blister strip packagings together with the instruction for medical use in the state and Russian languages place in a pack from cardboard bandbox.
On 5 packs place in group packing.
To Store storage conditions in the dry, protected from light place at
a temperature not higher than 25 S. Hranit in the places inaccessible for children!
3 years
not to apply a period of storage after the expiry date specified on packing.
Prescription status
According to the prescription
Dr. of Reddis Laboratoris Limited Producer 7-1-27 Amerpert, Hayderabad-500,016, India
the Name of the owner of the Registration certificate: The Dr. of Reddis Laboratoris Limited, India
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products
Representative office the Dr. of Reddis Laboratoris Limited in the Republic of Kazakhstan:
050057 Almaty, 22nd Line St., 45
ph. 8 (727) 3941688
fax: 8 (727) 3941294
To develop
of Ketorol Torgovoye medicine a name
Ketorol
Mezhdunarodnoye the unlicensed
name Ketorolak Lekarstvennaya
the Tablet form, film coated 10 mg
Structure
One tablet contains
active agent ketorolak a trometamina of 10 mg
excipients:
cellulose microcrystalline (type 102), starch corn prezhelatinizirovanny, starch corn, silicon dioxide anhydrous colloid, magnesium stearate
structure of a cover: Opadri 03K51148 green (hypro methyl cellulose (6cps), the titan E171 dioxide, triacetin/glycerin, gland (III) oxide yellow E172, FD & With No. 1 dye (diamond blue FCF, aluminum varnish of 11-13%)
the Description
of the Tablet of round shape, with a biconvex surface, film coated olive-green color, with S engraving on one party and a smooth surface on other party, diameter (8.20 & plusmn, 0.20) mm and thickness (3.50 & plusmn, 0.20) mm.
Pharmacotherapeutic group
Non-steroidal anti-inflammatory drugs. Derivatives of acetic acid.
The code of automatic telephone exchange M01AB15
the Pharmacological
Pharmacokinetics At properties intake ketorolak is well soaked up from digestive tract. The maximum concentration in blood plasma (0,7 1,1 mkg/ml) is reached in 40 minutes after reception on an empty stomach of a dose of 10 mg. Food rich with fats reduces Cmax and delays its achievement on 1 hour
Ketorol almost completely contacts proteins of plasma (& gt, 99%) at the expense of the small volume of distribution (& lt, 0.3 l/kg).
Time of achievement of equilibrium concentration at oral administration 24 hours when assigning 4 times a day. At intake of 10 mg it makes 0.39 0.79 mkg/ml.
Almost all substance circulating in blood plasma represents ketorolak (96%) or it pharmacological an inactive metabolite r-hydroksiketorolak.
Drug passes through a placental barrier for 10%. In small concentration it is found in breast milk of women. Passes through a blood-brain barrier badly.
Drug is metabolized mainly in a liver, contacting glucuronic acid, and is removed by kidneys. In urine about 92% of the entered drug dose, and 40% - in the form of metabolites, 60% - in the form of not changed substance are found. About 6% of the entered dose are removed with a stake. Metabolites have no essential analgesic activity.
At patients of senile age (65 years are more senior) the elimination half-life of a terminal phase, in comparison with that at persons of young age, increases till 7 o'clock (from 4.3 to 8.6 hours). The general plasma clearance, in comparison with persons of young age, can be reduced on average to 0.019 l/hour/kg.
At defeat of function of kidneys the removal of a ketorolak slows down what lengthening of elimination half-life and decrease in the general plasma clearance in comparison with young healthy volunteers testifies to. Speed of elimination decreases approximately in proportion to degree of a renal failure, except for patients with a heavy renal failure. At such patients the plasma clearance of a ketorolak becomes slightly higher than the damage of kidneys expected for this degree.
A pharmacodynamics
Ketorol the non-steroidal anti-inflammatory drug (NPVS) possessing soothing and anti-inflammatory action. The main mechanism of action of a ketorol, as well as at other NPVS, is shown in its pharmacological effect - inhibition of synthesis of prostaglandins. NPVS are most active on the periphery.
Ketorol has no sedative or anxiolytic effect, does not influence opioid receptors. Ketorol does not possess the oppressing action on a respiratory center and does not strengthen the respiratory depression and sedation caused by opioid analgetika. Ketorol does not cause medicinal dependence. After sharp drug withdrawal the symptoms of cancellation do not arise.
Indications
- stopping of pains in the postoperative period
- stopping of pains in muscles and joints
- a posttraumatic pain syndrome
- renal colic
the Route of administration and doses
Is appointed a short course of treatment and is applied within 7 days, it is not recommended for long-term treatment.
Adults
On 10 mg of each 4 6 hours. The daily dose should not exceed 40 mg.
For the patients accepting ketorol parenterally the general dose of drug at combination therapy should not exceed 90 mg a day.
Side effects
Often (more than 3%)
- a headache, dizziness, drowsiness
- edemas of face, shins, anklebones, fingers, a foot
- increase in body weight
is Less frequent (1 3%)
- skin rash, a purpura
- the increased perspiration
Seldom (less than 1%)
- an acute renal failure, a back pain with or without hamaturia and/or azotemias, a hemolytic uraemic syndrome (hemolytic anemia, a renal failure, thrombocytopenia, a purpura), frequent urination, increase or decrease in volume of urine, nephrite, swell renal genesis
- decrease in hearing, a ring in ears, a disorder of vision
- a bronchospasm or dispnoe, rhinitis, a laryngeal edema
- anemia, an eosinophilia, a leukopenia
- bleeding from a postoperative wound, nasal bleeding, rectal bleeding
- an anaphylaxis or anaphylactoid reactions (discoloration of face skin, skin rash, a small tortoiseshell, an itching of skin, a tachypnea, swell a century, periorbital swelled, an asthma, the complicated breath, weight in a thorax, goose breathing)
- aseptic meningitis (fever, a severe headache, spasms, muscle tension of a neck and/or back), hallucinations, a depression, psychosis
- exfoliative dermatitis (fever with a fever or without it, reddening, consolidation or peeling of skin, swelling and/or morbidity of palatine tonsils), a small tortoiseshell, Stephens-Johnson's syndrome, a Lyell's disease
- a paraglossa, fever.
Contraindications
- hypersensitivity to a ketorol, aspirin or to other NPVS
- aspirinovy asthma, a Quincke's disease
- a hypovolemia
- dehydration
- digestive tract erosive cankers
- round ulcers
- hypocoagulation, including hemophilia
- a liver, renal failure
- a hemorrhagic stroke
- bleedings, including after operation
- hemopoiesis disturbance
- before - and the operational period, because of high risk of bleeding
- chronic pains
- pregnancy and the period of a lactation
- children's and teenage age up to 18 years
the Simultaneous use of a ketorol with acetylsalicylic acid or other NPVS, calcium drugs, glucocorticoids, ethanol, corticotropin can lead Medicinal interactions to formation of ulcers of digestive tract and development of gastrointestinal bleedings.
Combined use with paracetamol increases nephrotoxicity, with a methotrexate gepato- and nephrotoxicity. Co-administration of a ketorol and methotrexate is possible only when using low doses of the last.
Probenetsid reduces plasma clearance and volume of distribution of a ketorol, increases its concentration in blood plasma and extends elimination half-life twice. The concomitant use of a ketorol and a probenetsid is contraindicated.
At combination with opioid analgetics, doses of the last can be significantly reduced.
Hypoglycemic effect of insulin and oral hypoglycemic drugs increases.
Joint appointment with Valproatum of sodium causes disturbance of aggregation of thrombocytes.
Ketorol increases concentration in blood plasma of verapamil and nifedipine.
When assigning with other nefrotoksichny medicines, including with gold drugs, risk of development of nephrotoxicity increases.
The medicines blocking canalicular secretion reduce clearance of a ketorol and increase its concentration in blood plasma.
Ketorol slightly reduces linking of warfarin with proteins. At a concomitant use with furosemide ketorol reduces diuretic effect of furosemide approximately by 20%.
At a concomitant use of a ketorol with drugs of lithium the concentration of lithium in plasma at the expense of inhibition of clearance of lithium kidneys some NPVS increases.
At a concomitant use of a ketorol and not depolarizing muscular relaxants the patients have a short wind.
At reception with antiepileptic drugs (Phenytoinum, carbamazepine) the frequency of attacks amplifies. At a concomitant use with psychotropic drugs (fluoxetine, tiotiksin, to alprazola) patients have hallucinations.
Co-administration and a pentoksifillina can increase risk of developing bleedings.
At simultaneous use with furosemide ketorol reduces diuretic effect of furosemide approximately by 20% therefore patients with heart failure need to appoint drug with care.
At at the same time use with APF inhibitors ketorol increases risk of a renal failure.
Special instructions
As NPVS reduce aggregation of thrombocytes, it is necessary to appoint ketorol with care the patient with disturbance of a coagulant system of blood.
Use for patients from the liver broken by function
At reception of a ketorol is possible increase in level of hepatic transaminases. Ketorol it is necessary to appoint a short course of treatment the patient with liver diseases.
Use for patients with impaired renal function
Ketorol is appointed the patient with impaired renal function with care under control of analyses of urine.
Use for patients of advanced age
At reception of a ketorol the side reactions at patients of advanced age meet more often, purpose of low doses of drug is necessary. The maximum doses should not exceed 60 mg for patients 65 years are more senior.
It is necessary to take the drug with an interval of 6 8 hours.
Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
As at patients when assigning a ketorol develop by-effects from the central nervous system (dizziness, drowsiness) and from sense bodys (decrease in hearing, a ring in ears, a disorder of vision), it is recommended to avoid performance of work, the requiring special attention and fast reaction.
Overdose
Symptoms: abdominal pains, nausea, vomiting, round ulcer of a stomach and duodenum, erosive gastritis, renal failure, metabolic acidosis.
Treatment: gastric lavage, administration of adsorbents (the coal activated), symptomatic treatment.
A form of release and packing
On 10 tablets in blister strip packaging from a film of the polyvinylchloride and printing aluminum foil varnished.
On 2 blister strip packagings together with the instruction for medical use in the state and Russian languages place in a pack from cardboard bandbox.
On 5 packs place in group packing.
To Store storage conditions in the dry, protected from light place at
a temperature not higher than 25 S. Hranit in the places inaccessible for children!
3 years
not to apply a period of storage after the expiry date specified on packing.
Prescription status
According to the prescription
Dr. of Reddis Laboratoris Limited Producer 7-1-27 Amerpert, Hayderabad-500,016, India
the Name of the owner of the Registration certificate: The Dr. of Reddis Laboratoris Limited, India
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products
Representative office the Dr. of Reddis Laboratoris Limited in the Republic of Kazakhstan:
050057 Almaty, 22nd Line St., 45
ph. 8 (727) 3941688
fax: 8 (727) 3941294
To develop