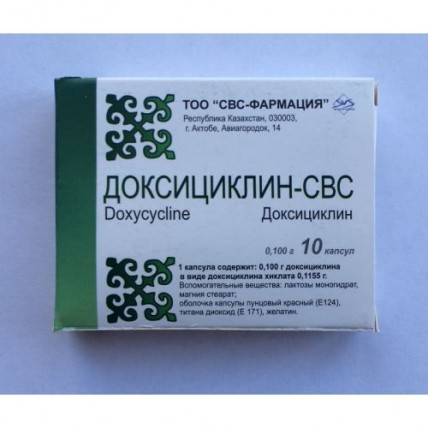
Doxycycline-SVS 100 mg, 10 capsules
- $8.00
Out Of Stock
Instruction for medical use of medicine Doxycycline – SVS the Trade name Doxycycline – SVS the International unlicensed name Doxycycline Dosage Form of the Capsule of 0.1 g Structure One capsule contains active agent – doxycycline (in the form of hiklat doxycycline) - 0.1 g excipients: lactoses monohydrate, magnesium stearate Structure of the capsule: a lid - crimson red (E 124), the titan dioxide (E 171), gelatin, the body - crimson red (E 124), the titan dioxide (E 171), gelatin. The description Solid gelatin capsules of No. in size 3 cylindrical forms with the body and a lid of pink color. Contents of capsules – powder of yellow color. Pharmacotherapeutic group Antibacterial drugs for system use. Tetracyclines. Doxycycline. The ATX J01AA02 code the Pharmacological Pharmacokinetics Docsitsiklin properties is quickly soaked up from a digestive tract in proportion to the accepted dose. This process can slow down the food contents, especially dairy products and drugs containing ions of metals. After intake of 200 mg of drug reaches the maximum concentration in plasma making 2.6 mkg/ml doxycycline in 2 hours. About 50% of doxycycline are metabolized in a liver. Doxycycline contacts proteins of serum for 80 – 90%. Biological elimination half-life of drug makes from 18 to 22 hours and is extended approximately till 25 o'clock at patients with a renal failure. Thanks to long elimination half-life it is possible to take the drug once a day. Doxycycline is well dissolved in fats. Easily gets into the majority of biological liquids and body tissues. Drug reaches high concentrations in watery moisture of an eye, in a prostate, ovaries, a uterus, a bladder, bile, a liver, muscles, rudiments of teeth and bones, in separated a bronchial tree, easy, lymph nodes, near-nasal bosoms, palatine tonsils. Passes through a placenta. Gets into maternal milk. At patients with normal function of kidneys about 40% of drug it is removed in not changed view with urine, and the rest of drug is removed with a stake, mainly in the form of metabolites. Doxycycline is not exposed to considerable cumulation in an organism of patients with a renal failure. Biological elimination half-life and AUC value of doxycycline do not change. Correction of a dosage is not required from patients with a renal failure because at such patients the concentration of doxycycline in bile increases therefore its removal with a stake increases. The hemodialysis does not influence biological elimination half-life of drug from serum. The pharmacodynamics of Docsitsiklin-SVS is the antibiotic belonging to group of tetracyclines. Possesses a broad spectrum of activity concerning gram-negative and gram-positive bacteria and also protozoa. The bacteriostatic mechanism of effect of drug consists in slowing down of synthesis of protein at the ribosomalny level. Antibacterial activity. In vitro doxycycline possesses antibacterial action in the relation: - gram-negative bacteria of Neisseria gonorrhoeae, Campylobacter granulomatis, Haemophilus ducreyi, Haemophilus influenzae, Pasteurella pestis, Pasteurella tularensis, Vibrio cholerae, Bartonella bacilliformis, Brucella spp., the changeable sensitivity is shown: Escherichia coli, Klebsiella spp., Enterobacter aerogenes, Shigella spp., Mima spp., Herella spp., Bacteroides spp. - gram-positive bacteria of Streptococcus pyogenes, Streptococcus pneumoniae, Streptococcus faecalis, Streptococcus faecium, Staphylococcus aureus, α-hemolytic streptococci of the viridans group - Other microorganisms of Rickettsia, Chlamydia psittaci, Chlamydia trachomatis, Mycoplasma pneumoniae, Ureaplasma urealyticum, Borrelia recurrentis, Treponema pallidum, Clostridium spp., Fusobacterium fusiforme, Actinomyces spp., Bacillus anthracis, Propinibacterium acnes, Entamoeba spp., Balantidium coli. Plasmodium falciparum. Doxycycline resistance is observed among many strains, especially among gram-positive bacteria. This phenomenon is characterized by heterogeneity since big differences of stability of bacteria of different regions of the world are often observed. The mechanism of stability to doxycycline is connected with decline in the ability of drug to penetrate in a bacterial cell. This stability is caused by presence at a cell of transferable R-plazmidov. Streptococcus pneumoniae strains resistant to doxycycline show also other tetracyclines resistance, and is frequent to penicillin and macroleads (cross stability). Synergism of action between doxycycline and macroleads is observed. Indications - infections of upper and lower airways (tonsillitis, pharyngitis, average otitis, sinusitis, bronchitis, pneumonia) caused by Mycoplasma pneumoniae, Chlamydia pneumoniae - as alternative drug in patients with hypersensitivity to penicillin in the respiratory infections caused by Haemophilus influenzae, Klebsiella spp., Streptococcus pneumoniae - acute and persistent infections of uric ways (the pyelonephritis, cystitis, an urethritis caused by Chlamydia trachomatis, Ureaplasma urealyticum) - the infections of soft tissues caused by microorganisms, for example Clostridium spp., sensitive to drug, Propinibacterium acnes - the infections of a digestive tract caused by strains of Escherichia coli, Entamoeba histolytica, Shigella spp., Vibrio cholerae, Clostridium spp. - the infections of an urinogenital system caused by Chlamydia trachomatis - treatment of gonorrhea and syphilis - the conjunctivitis caused by Chlamydia trachomatis - other infections caused by the microorganisms sensitive to drug: an inguinal granuloma (Calymmatobacterium granulomatis), a venereal ulcer (Haemophilus ducreyi), trachoma (Chlamydia trachomatis), an ornithosis (Chlamydia psittaci), a brucellosis (Brucella spp), a bartonellosis (Bartonella bacilliformis), plague (Yersibia pestis), a tularemia (Francisella tularensis), infections caused by Campylobacter fetus - prevention of the malaria caused by Plasmodium falciparum in the persons making short trips (it is less than 4 months) to regions where the malaria strains resistant to chloroquine are widespread. Attention! Before an initiation of treatment it is necessary to define sensitivity of the allocated activator to doxycycline. Treatment can be begun before obtaining result of sensitivity. After receiving an antibiotikogramma there can be necessary the corresponding replacement of drug. To Apply a route of administration and doses strictly on doctor's orders in order to avoid complications. Doxycycline is appointed only in the presence of absolute indications. Adults: In the first day of treatment appoint usually 200 mg of doxycycline in 1 reception or divide into 2 receptions – to 100 mg there are each 12 hours, and then pass to a maintenance dose – 100 mg a day. In heavy infections during the entire period of treatment appoint 200 mg of doxycycline a day. Course of treatment: at administration of drug inside depending on features of the course and severity of a disease the course of treatment is equal 7-10 days. Side effects Often - loss of appetite, nausea, vomiting, a diarrhea, a glossitis, swallowing difficulty, a coloenteritis, inflammatory changes in area of an anus (anus) - a photosensitization, a photophobia - allergic reactions in the form of rash, dermatitis, urticaria, it is rare - hemolytic anemia, thrombocytopenia, a neutropenia, an eozinifiliya, - hepatotoxicity, increase in hepatic transaminases, bilirubin in blood serum - increase in intracranial pressure, a headache, dizziness, sonitus Very seldom - increase in concentration of urea in blood - a system lupus erythematosus, a Lyell's disease, a mnogoformny erythema, a syndrome Stephens-Johnson, toxic necrosis of epidermis, an anaphylaxis - a pericarditis, tachycardia in case of the expressed undesirable symptoms the use of drug needs to be stopped immediately. Contraindications - hypersensitivity to doxycycline, other tetracyclines or to any substance which is a part of drug - a heavy liver and renal failure - children's age up to 18 years - pregnancy and the period of a lactation - the hereditary lactose intolerance, galactoses, a lactose intolerance Medicinal interactions Doxycycline strengthens effect of anticoagulative drugs, derivative coumarin and sulphonylurea, during use of doxycycline there can be necessary a reduction of a dose of these drugs. Strengthens nephrotoxic action of a metoksifluran and other potentially nephrotoxic drugs. Strengthens toxic action of a methotrexate and cyclosporine A. Weakens antibacterial effect of penicillin and other antibiotics of bactericidal action. Reduces efficiency of oral contraceptives. During treatment, doxycycline recommends use of additional effective methods of contraception. The drugs inducing microsomal enzymes, such as barbiturates, carbamazepine, diphenylhydantoin and also alcohol accelerate doxycycline metabolism therefore its biological elimination half-life can be reduced and decrease therapeutic action. At the patients taking the above-stated drugs it is necessary to consider the possibility of increase in a daily dose of doxycycline. At simultaneous use of doxycycline and theophylline, by-effects from digestive system are more often shown. Doxycycline affects results of definition of glucose, urobilinigen, protein and catecholamines in urine. Doxycycline should not be accepted along with compounds of calcium, aluminum, magnesium and iron and also with activated carbon or holestiraminy since they reduce its absorption in a digestive tract. Use of a metoksifluran for an inhalation anesthesia at the patients accepting tetracyclines can lead to heavy injuries of kidneys. Doxycycline can increase cyclosporine level in blood serum, in case of a concomitant use of drugs. Simultaneous use of tetracyclines and retinoids (acetretinas, izotretinoin, tretinoin) can promote increase in intracranial pressure. Doxycycline increases the level of digoxin and compounds of lithium in serum. Simultaneous use of doxycycline and diuretics can strengthen their nephrotoxic action. The special instructions Doxycycline to a lesser extent, than other tetracyclines, forms strong complex connections with calcium in all bone tissues. However its use during development of teeth (last trimester of pregnancy, the perinatal period, the early childhood) can lead to coloring and injury of teeth or an underdevelopment of a skeleton. During treatment the overgrowth of steady microorganisms, for example Candida can arise doxycycline. If there is an infection with steady microorganisms, doxycycline it is necessary to cancel and begin the corresponding treatment. Doxycycline, also as well as other antibiotics of a broad spectrum of activity can cause the pseudomembranous colitis caused by development of sticks of Clostridium difficile. Therefore patients who during treatment by doxycycline or right after its termination complain of a diarrhea should be examined for the purpose of detection of this disease and, in case of confirmation of the diagnosis, to undertake the corresponding treatment (drug withdrawal, Vancomycinum). Administration of drugs braking a vermicular movement of the intestines or other means fixing actions is contraindicated. During treatment it is necessary to avoid stay to the sun or influences of artificial UV-radiation (for example, a sunbed) in connection with danger of development of photodermatoses. Doxycycline is not exposed to a considerable kumulirovaniye correction of dosing in this regard is not required from patients with a renal failure. To apply carefully to patients with a liver failure and to the patients taking other gepatotoksichny drugs. To patients, it is long accepting doxycycline, control of function of a liver, kidneys and a picture of blood Pregnancy and a lactation has to be carried out Docsitsiklin gets through a placenta. Can cause long-term discoloration of teeth, an enamel hypoplasia, suppression of growth of bones of a skeleton of a fruit. The feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms does not affect ability to run the vehicle. Overdose overdose Symptoms doxycycline are fever, face reddening, dizziness, collapse can sometimes develop. Treatment: drug withdrawal, gastric lavage, control of the main vital functions (pulse, breath) and in case of need - symptomatic treatment. Use of a hemodialysis for elimination of drug from blood is inefficient. A form of release and packing On 10 capsules in blister strip packaging from a film of the polyvinylchloride brand and/or printing aluminum foil varnished. On 1 blister strip packaging together with the instruction for use in the state and Russian languages place in a pack from cardboard for a retail container. Storage conditions In the dry, protected from light place, at a temperature not over 25 of 0C. To store out of children's reach! A period of storage 3 years not to use drug after the expiration date specified on packing. Prescription status According to the prescription SVS-Pharmation LLP Producer, Legal address: 030012 Republic of Kazakhstan, Aktobe, Sankibay Ave of the batyr 171, B the location Address: 030030 Republic of Kazakhstan, Aktobe, Mr. Aviagorodok, 14 Owner of the registration certificate of SVS-Pharmation LLP, 030012 Republic of Kazakhstan, Aktobe, Sankibay Ave of the batyr 171,B.
To develop
To develop