Description
The instruction
on medical use
of medicine
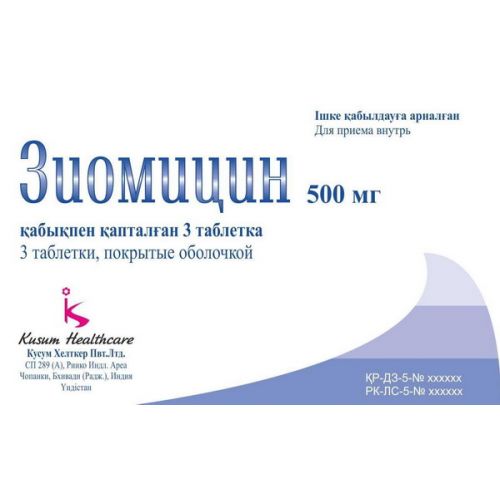
Ziomitsin
the Trade name
Ziomitsin
Mezhdunarodnoye the unlicensed
name Azithromycin Dosage Form
of the Tablet, coated 250 and 500 mg
Structure
One tablet contains
active agent – dihydrate azithromycin in terms of azithromycin of 250 mg and 500 mg,
excipients: cellulose microcrystalline (Avitsel PH 101), sodium of a kroskarmelloz (Ac-Di-Sol), sodium lauryl sulfate (Stepanol WA100), povidone (Plasdone K 90), talc purified magnesium stearate, Opadry 04B520005 covering Yellow: (a gipromelloza 15 sr, the titan E171 dioxide, makrogol/PEG 400, gland (III) oxide yellow E172)
the Description
of the Tablet, coated yellow color, a capsulovidny form, with the And 250 logo on one party and smooth on the other hand (for a dosage of 250 mg).
Tablets, coated yellow color, a capsulovidny form, with the And 500 logo on one party and smooth on the other hand (for a dosage of 500 mg).
Pharmacotherapeutic group
Antibacterial agents for system use. Macroleads.
ATC J01FA10 code
the Pharmacological
Pharmacokinetics Azithromycin properties is quickly soaked up from an alimentary canal that is caused by firmness in acidic environment and lipophilicity. The bioavailability is 37%. The maximum concentration in blood serum is reached in 2.5-3 h and makes 0.4 mg/l at intake of 0.5 g of azithromycin. Drug well gets into airways, bodies and fabrics of an urogenital highway, in particular into a prostate, skin and soft tissues. Concentration of drug in fabrics and cells 10-15 times higher, than in blood serum. High concentration in fabrics and the long period poluvyvedennya are caused by low linking of azithromycin with serum proteins and also ability to get into eukaryotic cells and to concentrate in the environment with a low rn which surrounds lysosomes. It determines, in turn, the large volume of imaginary distribution (31.1 l/kg) and high plasma clearance. It is proved that phagocytes deliver drug to the place of an infection where release it. Good penetration of azithromycin into cells and accumulation in phagocytes with which it is transported in inflammation cells renders assistance to antimicrobic activity of drug. Despite high concentration in phagocytes, azithromycin essentially does not influence their function. In inflammation cells drug is in bactericidal concentration within 5–7 days after reception of the last dose that allowedshort (the 3rd and 5-day) courses of treatment.
Elimination of azithromycin comes from blood serum in two stages: elimination half-life makes 14–20 h between 8 and 24 h after administration of drug and 41 h – in the range from 24 up to 72 h that allows to use drug once a day.
Meal considerably changes pharmacokinetics: the maximum concentration of drug in blood plasma decreases by 52%. About 35% of azithromycin are metabolized in a liver by demethylation, metabolites which are formed, inactive. More than 59% of drug are removed with bile and about 4.5% – with urine in not changed look.
The pharmacodynamics
Azithromycin is the representative of group of makrolidny antibiotics – azaleads which has a wide range of antimicrobic action. It oppresses biosynthesis of proteins of microorganisms, contacting a ribosome 50S-subunit. Active concerning a number of gram-positive bacteria: Streptococcus pneumoniae, S. pyogenes, S. agalacticae, streptococci of the C, F and G, Staphylococcus aureus and S. epidermidis groups. Does not affect the gram-positive bacteria resistant to effect of erythromycin. Effective rather gram-negative microorganisms: Haemophilus influenzae, H. parainfluenzae and H. ducrei, Moraxella catarrhalis, Bordetella pertussis and B. parapertussis, Neisseria gonorrhoeae and N. meningitidis, Brucella melitensis, Helicobacter pylori, Gardnerella vaginalis. Affects sensitive anaerobic microbes: Clostridium spp., Peptostreptococcus spp., Peptococcus spp. Besides, is effective rather intracellular and other microorganisms, including: Legionella pneumophila, Chlamydia trachomatis and C. pneumoniae, Mycoplasma pneumoniae, Ureaplasma urealyticum, Listeria monocitogenes, Treponema pallidum, Borrelia burgdorferi.
Indications
– infections the ENT SPECIALIST – bodies (synovitis, pharyngitis, tonsillitis, average otitis)
– respiratory infections: an acute bronchitis and interstitial and alveolar pneumonia
– the diseases of a stomach and a duodenum caused by Helicobacter pylori
– an infection of skin and soft tissues (the chronic migrating erythema (initial stage of a disease of Lyme), erysipelatous inflammation, impetigo, secondary pyodermatoses)
– infections, sexually transmitted (including the urogenital clamidiosis, an uncomplicated urethritis and a cervicitis caused by Chlamydia trachomatis)
it is necessary to accept the Route of administration and doses of Ziomitsin® surely for an hour to or two hours after a meal as the concomitant use with food reduces azithromycin absorption. The drug is taken once a day.
Adults and children with body weight over 50 kg:
in infections of ENT organs and airways, skin and soft tissues (except the chronic migrating erythema): 500 mg (2 tablets on 250 mg or 1 tablet on 500 mg disposable) in day for 3 days. A course dose – 1.5 grams.
At the chronic migrating erythema: 1 time a day for 5 days, 1 – y day – 1 g (4 tablets on 250 mg or 2 tablets on 500 mg), then – on 500 mg (2 tablets on 250 mg or 1 tablet on 500 mg) from the 2nd to the 5th day.
In infections which are transmitted sexually: 1 g disposable (4 tablets on 250 mg or 2 tablets on 500 mg).
In a peptic ulcer of a stomach and duodenum: 1 g (4 tablets on 250 mg or 2 tablets on 500 mg) in day for 3 days together with anti-secretory means and other medicines on doctor’s orders.
Use for children:
Ç¿«¼¿µ¿¡® 250 mg are recommended to appoint to children with body weight from 25 to 50 kg, at the rate of 10 mg / 1 in body weight kg, for 3 days.
In case of the admission of reception of 1 dose of drug, it is necessary to accept the passed dose as soon as possible, and the following – with intervals at 24 o’clock.
People of advanced age and at patients with renal failures have no need to change dosing.
Dosing for patients from the liver broken by function.
Dose adjustment as azithromycin is substantially metabolized in a liver is necessary.
Dosing for patients of advanced age.
If function of a liver and kidneys is not broken, there is no need for dose adjustment.
Side effects
Often
– nausea, vomiting, diarrhea, an abdominal pain
Seldom
– nausea, a meteorism, a constipation
– tranzitorny rise in level of aminotransferases of a liver, bilirubin,
eosinophils in blood. Indicators return to norm in 2-3 weeks after the end of therapy
– cholestatic jaundice, hepatitis, pseudomembranous colitis
– reactions of hypersensitivity (reddening, skin rash, an itching,
a Quincke’s disease, photosensitivity)
Is extremely rare
– fatigue, a headache, dizziness
– anaphylactic and skin reactions, such as reddening, skin rash, an itching, Stephens-Johnson’s syndrome, a toxic epidermal necrolysis, an acute anaphylaxis
– disorders of taste and sense of smell, temporary decrease in hearing, deafness and sonitus
– paresthesias, a faint
– concern, nervousness, insomnia
– heartbeat, arrhythmias, thorax pain
Isolated cases
– arthralgias
– interstitial nephrite
– an acute renal failure
of the Contraindication
– hypersensitivity to azithromycin, makrolidny antibiotics or other components of drug
– liver diseases
– diseases of kidneys
– pregnancy and the period of a lactation
– children’s age up to 12 years
Medicinal interactions
Antiacid means slow down azithromycin absorption. It is recommended to adhere not less than a two-hour interval between administration of drug and an antacid. It is necessary to pay attention to a concomitant use of drug with theophylline, terfenadiny, warfarin, carbamazepine, Phenytoinum, triazolamy, digoxin, ergotamine, cyclosporine as makrolidny antibiotics can enhance effect of above-mentioned drugs. Unlike the majority of macroleads, azithromycin does not contact P450 cytochrome complex enzymes. To this time the interaction of azithromycin with the specified drugs was not observed.
Special instructions
It was reported that sometimes there were serious allergic reactions, such as hypostasis and anaphylaxis (sometimes with a lethal outcome). Sometimes such reactions during intake of azithromycin repeated and demanded longer observation and treatment.
The break between intake of azithromycin and antiacid drugs is necessary at least 2 hours.
It is recommended to make observations of signs of superinfection at irresponsive people, especially with fungus diseases.
Use in a renal failure. There is no need to adjust a dosage at patients with a renal failure from moderated to moderate severity (glomerular filtration rate of 10-80 ml/min). It is recommended to appoint drug with care to patients with a heavy renal failure (glomerular filtration rate
Use in a liver failure. As azithromycin is metabolized in a liver and removed with bile, the patients having serious diseases of a liver should not take this drug. Researches on treatment of such patients using azithromycin were not conducted. In case of developing of a heavy renal failure the treatment by azithromycin needs to be stopped.
Patients with neurologic and mental disorders need to appoint azithromycin with care.
Azithromycin is not appointed for treatment of an infection of burn wounds.
Azithromycin tablets, coated, are not suitable for treatment of heavy infections in cases when fast and strong increase in concentration of an antibiotic in blood is necessary.
The feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
during treatment needs to be careful during the driving of motor transport and occupation other potentially dangerous types of activity demanding the increased concentration of attention and speed of psychomotor reactions.
Overdose
Symptoms: reversible hearing loss, severe nausea, vomiting and diarrhea
Treatment: gastric lavage and general maintenance therapy.
A form of release and packing
of the Tablet of 250 mg
On 6 or 21 tablet in blister strip packaging. One blister strip packaging No. 6 or No. 21 together with the instruction for medical use in the state and Russian languages in cardboard packing.
Tablets of 500 mg
On 3 tablets in blister strip packaging. One blister strip packaging No. 3 together with the instruction for medical use in the state and Russian languages in cardboard packing.
To Store storage conditions in the dry, protected from light place, at a temperature not above 25ºС.
To store out of children’s reach!
3 years
not to use a period of storage after the termination of the expiration date specified on packing
Prescription status
According to the prescription
the Producer Kusum Heltker Pvt.
Ltd., JV 289 (A), Riiko Indl. Chopanki’s Area, Bkhivadi (Raj.), India
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods):
Representative office of “Kusum Heltker Pvt. Ltd.” Association, India in RK
Almaty, Tulebayev St., 38
ph. / fax: 273-93-74, 273-68-10
the e-mail address:
Office-kusumhealthcare@intelsoft.kz
Additional information
| Ingredient |
|---|














Reviews
There are no reviews yet.