Description
The instruction for use
medicine for experts
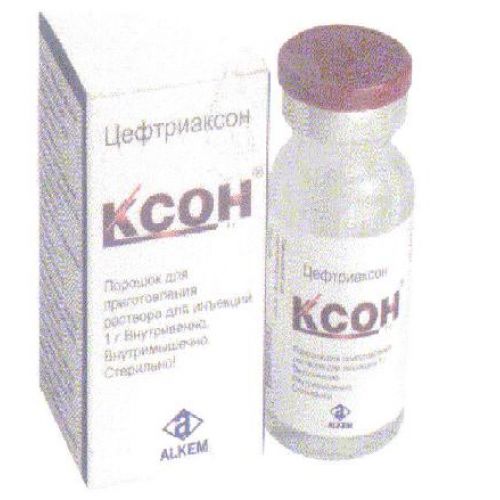
of KCOH XONE
the Trade name
Kson
Mezhdunarodnoye the unlicensed
name Tseftriakson Lekarstvennaya a form
Powder for preparation of solution for injections
Structure
Active agent – tseftriakson (in the form of sodium salt) 1 g
the Description
From white till yellowish-orange color crystal powder
Pharmacotherapeutic group
Others beta laktamnye antibiotics. Cephalosporins.
J01DA13
Pharmacological
Pharmacokinetics Absorption properties and distribution: at parenteral administration tseftriakson well gets into fabrics and liquids of an organism. The area under a curve concentration time at intravenous and intramuscular administration match. The bioavailability of a tseftriakson after intramuscular introduction is 100%. At intravenous administration tseftriakson quickly diffuses in interstitial liquid where during 24 h bactericidal concentration concerning sensitive bacteria are maintained.
Tseftriakson reversibly contacts albumine. Extent of binding is inversely proportional concentration of a tseftriakson in blood serum (for example, at concentration in blood serum less than 100 mg/l linking of a tseftriakson with proteins makes 95%, and at concentration of 300 mg/l – 85%). Thanks to lower content of albumine in interstitial liquid the concentration of a tseftriakson in it is higher, than in blood serum.
Tseftriakson gets through a blood-brain barrier. At newborns and children at inflammation of a meninx tseftriakson gets into cerebrospinal fluid. In bacterial meningitis the concentration of a tseftriakson in cerebrospinal fluid averages 17% of its concentration in blood serum that is about 4 times more, than in aseptic meningitis. In 24 h after intravenous administration of drug in a dose of 50-100 mg/kg of body weight the concentration of a tseftriakson in cerebrospinal fluid exceeds 1.4 mg/l. At adults in meningitis in 2-25 h after administration of drug in a dose of 50 mg/kg of body weight the concentration of a tseftriakson significantly exceeded the minimum overwhelming concentration.
Metabolism and removal: elimination half-life at healthy volunteers makes about 8 h. Newborns till 8th day have lives and at elderly people 75 years are more senior average elimination half-life makes about 16 h. At adult 50-60% of a tseftriakson 40-50% are removed in not changed view with urine, – in not changed view with bile. At newborns about 70% of the entered dose it is removed by kidneys. At intake tseftriakson it biotransformirutsya in an inactive metabolite under the influence of intestinal microflora.
In moderate renal failures or a liver at adults the pharmacokinetics of a tseftriakson almost does not change, elimination half-life is slightly extended. It is connected with the fact that at patients with renal failures the removal of a tseftriakson with bile kompensatorno increases, and at patients with abnormal liver functions the removal of a tseftriakson kidneys kompensatorno amplifies.
A pharmacodynamics
the Tsefalosporinovy antibiotic of the III generation for parenteral use. Has bactericidal effect due to oppression of synthesis of a cellular membrane of bacteria. In vitro suppresses growth of the majority of gram-positive and gram-negative microorganisms. Drug is resistant to action beta laktamaz (both penicillinases, and tsefalosporinaza).
Kson is active concerning aerobic gram-positive bacteria: Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus viridans, Streptococcus bovis, aerobic gram-negative bacteria: Aeromonas spp., Alcaligenes spp., Moraxella/Branhamella/catarrhalis, Citrobacter spp., Enterobacter spp. (some strains of a rezistentna), Escherichia coli, Haemophilus ducreyi, Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella spp. (including. Klebsiella pneumoniae), Moraxella spp, Morganella morganii, Neisseria gonorrhoeae, Neisseria meningitidis, Plesiomonas shigelloides, Proteus mirabilis, Proteus vulgaris, Providencia spp., Pseudomonas aeruginosa (some strains are steady), Salmonella spp. (including Salmonella typhi), Serratia spp. (including Serratia marcescens), Shigella spp., Vibrio spp. (including Vibrio cholerae), Yersinia spp. (including Yersinia enterocolitica), anaerobic bacteria: Bacteroides spp. (including some strains of Bacteroides fragilis), Clostridium spp. (including Clostridium difficile), Fusobacterium spp. (except Fusobacterium mostiferum, Fusobacterium varium), Peptococcus spp., Peptostreptococcus spp.
Are resistant to drug Methicillinum – resistant strains of Staphylococcus spp., the majority of strains of Enterococcus spp. (including Enterococcus faecalis), some strains of Enterobacter spp., some strains of Pseudomonas aeruginosa, some strains of Bacteroides spp. producing beta lactamazu (including Bacteroides fragilis).
Treponema pallidum is sensitive to a kson. Clinical data confirm high efficiency of a kson in primary and secondary syphilis.
It is necessary to apply the disks containing kson to determination of sensitivity of microorganisms (certain strains of activators can be resistant to classical cephalosporins).
Indications
the Bacterial infections caused by sensitive microorganisms:
– peritonitis
– sepsis
– meningitis
– infections of abdominal organs (inflammatory diseases of digestive tract, biliary tract, including a cholangitis, an empyema of a gall bladder)
– diseases of upper and lower airways (including pneumonia, abscess of lungs, a pleura empyema)
– infections of bones and joints
– infections of skin and soft tissues
– infections of urinary tract (including pyelonephritis)
– gonorrhea
– contaminated wounds and burns
Prevention of a postoperative infection. Infectious diseases at persons with the weakened immunity.
The route of administration and doses
administer the Drug intramusculary or intravenously.
For adults and children 12 years an average daily dose are more senior makes 1-2 g of a tseftriakson of 1 times a day. In hard cases or in infections which causative agents have sensitivity to a tseftriakson it is possible to increase a daily dose to 4 g.
For newborns (to two-week age) the dose makes 20-50 mg/kg/days.
For babies and children up to 12 years the daily dose makes 20-80 mg/kg. Children with body weight have 50 kg and apply doses to adults more.
It is necessary to appoint a dose more than 50 mg/kg of body weight in the form of intravenous infusion. Duration of a course of treatment depends on the nature of a disease.
In bacterial meningitis at babies and children of younger age the initial dose makes 100 mg/kg of 1 times a day. The maximum daily dose – 4 g.
For treatment of gonorrhea the dose makes 250 mg, once intramusculary.
For prevention of infections in the preoperative and postoperative periods in 30-90 min. prior to operation enter 1-2 g of a tseftriakson.
In a renal failure (clearance of creatinine less than 50 ml/min.) the daily dose of a kson should not exceed 1 g. In the profound abnormal liver functions and kidneys and also at the patients who are on a hemodialysis it is necessary to watch concentration of a kson in blood plasma since at them the speed of its discharge can decrease.
Rules of preparation and administration of drug
For preparation of solution for intramuscular introduction of 1 g of powder part 3.5 ml of 1% of solution of lidocaine. Solution is entered deeply into a gluteus. The received solution cannot be entered intravenously!
For preparation of solution for intravenous administration of 1 g of powder part 10 ml of the sterile distilled water and enter intravenously slowly within 2-4 min.
For preparation of solution for intravenous infusion of 2 g of powder it is necessary to part about 40 ml of solution free from calcium, for example: 0.9% solution of sodium of chloride, 5% solution of glucose, 10% solution of glucose, 5% levuloza solution. Duration of intravenous infusion – 30 min.
Side effects
– about 2%: diarrhea, nausea, vomiting, stomatitis, a glossitis, development of a psevdokholetiaz, an eosinophilia, a leukopenia, a granulocytopenia, hemolytic anemia, thrombocytopenia
– about 1% is possible: a dieback, allergic dermatitis, a small tortoiseshell, hypostasis, a multiformny erythema
– seldom: a headache, dizziness, increase in activity of enzymes of a liver, pain in right hypochondrium, fever, anaphylactic reactions, an oliguria, increase in content of creatinine in blood serum, mycoses of anogenitalny area, candidiasis of genitals, an oliguria
– in isolated cases: fibrillation disturbance, a pseudomembranous coloenteritis
After intravenous administration in certain cases: phlebitis, an itching in the place of an injection (this phenomenon can be avoided at slow / during 2-4 mines / administration of drug).
Side effects, as a rule, of a tranzitorna also disappear after drug withdrawal.
Contraindications
– hypersensitivity to a tseftriakson and penicillin
– pregnancy (I trimester) and the lactation period
– cholelithiasis
– existence in the anamnesis of a coloenteritis and bleeding.
Medicinal interactions
Kson and aminoglycosides have synergism concerning many gram-negative bacteria, however at simultaneous introduction with ftorkhinolona, Vancomycinum, rifampicin (but not in one syringe) the risk of crystallization in uric ways increases. At simultaneous use with loopback diuretics (for example, furosemide), disturbances of functions of kidneys are not observed. At simultaneous introduction with Euphyllinum drops out in a deposit.
Kson pharmaceutical is incompatible with the solutions containing other antibiotics.
The special
instructions Drug it is used only in the conditions of a hospital. At Kson’s use it is necessary to consider a likelihood of development of an acute anaphylaxis. In case of its development it is necessary to enter immediately intravenously adrenaline, and then glucocorticoid means.
In a simultaneous heavy renal and liver failure it is regularly necessary to define concentration of drug in blood plasma. At long-term treatment it is regularly necessary to control a pattern of peripheral blood, indicators of a functional condition of kidneys and to gradually reduce a drug dose.
It is necessary to consider that sometimes at ultrasonography of a gall bladder the existence of the shadow indicating adjournment of precipitation is noted. This symptom disappears after the termination or the temporary termination of therapy tseftriaksony. At a pain syndrome in similar cases the surgical intervention is not required, carry out conservative treatment. Alcohol intake during therapy has similar effect.
Use in pediatrics
Kson is capable to force out the bilirubin connected with blood serum albumine. Therefore at newborns with a hyperbilirubinemia and, especially at premature newborns, use of a tseftriakson demands extra care.
Pregnancy and a lactation
Drug is contraindicated to use in the I trimester of pregnancy. Kson’s use in II and III trimesters of pregnancy and in the period of a lactation perhaps only if the expected advantage for mother exceeds potential risk for a fruit or the child. When assigning in the period of a lactation it is necessary to cancel breastfeeding.
Influence on ability to driving of motor transport and to control of mechanisms
it is necessary to resolve the Issue of a possibility of occupations by potentially dangerous types of activity only after assessment of individual reaction of the patient to drug.
Overdose
Symptoms: strengthening of side effects.
Treatment: symptomatic. The hemodialysis and peritoneal dialysis are inefficient.
Form of release and packing
Powder for preparation of solution for injections, on 1 g in the glass bottle corked by a rubber bung, the pressed-out aluminum cap and closed by the protecting plastic cap. On 1 bottle together with the instruction for use place in a cardboard box.
Storage conditions
Drug should be stored in the dry, protected from light place at a temperature not above +25 °C.
To store drug out of children’s reach!
3 years
not to use an expiration date after an expiration date.
Alkem Laboratories Ltd Producer Alkem House Devashish, Senapati Bapat Marg, Lower Parel, Mumbai – 400,013
To develop prescription status According to the prescription
Additional information
| Ingredient |
|---|

















Reviews
There are no reviews yet.